 COVID-19 Testing
COVID-19 Testing
It is clear that testing for COVID-19 is of critical importance to the country as a whole to allow for social planning, but it is also important for the individual for the many obvious reasons. Testing for COVID-19 is available now in the New Orleans area, please see below for how to obtain testing. However, before testing, please read this page completely to gain a full understanding of what information the test provides but also to understand what is not known about COVID-19 testing.
Who should consider testing?
- If one has had a recent flu-like illness and recovered, but the illness is suspicious for possible COVID-19, one should consider serology testing for antibodies (see below) to determine if it was COVID-19 and evaluate for possible immunity to COVID-19.
- If one has had close contact with someone with positive testing for COVID-19, even if one has not had symptoms, one should consider serology testing for antibodies (see below) to determine if one had an asymptomatic infection and evaluate for possible immunity to COVID-19.
- If one currently has flu-like symptoms suggestive of possible COVID-19, testing for the virus (RT-qPCR test – see below) in an ideal world they should be tested. However, given the lack of available supplies, testing for the virus is currently advised only for those with severe symptoms or those at increased risk of bad outcomes.
Testing Options:
Where to get viral (RNA) testing for acute illness:
Ready Responders
Ready Responders will come to your home for free testing if your are acutely ill with symptoms consistent with COVID-19.
Call: (504) 370-9966
Where to get antibody (serology) testing:
Innovative Risk Management Services
2714 Canal Street
Suite 102
New Orleans, LA 70119
(504) 309-2104
Please call first for an appointment before going to Innovative, otherwise you will not be seen. Also, please observe usual COVID-19 precautions by wearing a mask when you go and respect 6′ social distancing.
The COVID testing samples will be obtained at Innovative but the actual sample testing is performed by LabTcch, a CAP accredited lab, the highest standard for lab certification in the country. This is not a fly-by night lab – the test results are reliable.
See also:
COVID-19 (CAM Tx) – Explores ways to possibly reduce the severity of COVID-19 infection if it develops.
.
COVID-19 and SARS-CoV-2
COVID-19 (COrona VIrus Disease 2019) refers to the infection by the corona virus named SARS-CoV-2 which stands for “Severe Acute Respiratory Syndrome COrona Virus-2,” with the original SARS coronavirus (SARS-CoV-1) identified in 2003 related to another outbreak first identified in China.
What percentage of people infected with SARS-CoV-2 have symptoms of COVID-19?
This is the big question and the answer is not yet known. However, a recent study of the infection in China in January 2020 provides some estimates. The study suggests that 86% of SARS-CoV-2 infections went without symptoms. or with very mild symptoms. Of interest, it was also noted that people who never developed symptoms of infection were only 55% as contagious as those who did develop symptoms. The lack of adequate testing in this country does not allow for estimates here yet.
Incubation period of COVID-19
To guide the decision as to when to test, it is important to understand the incubation period of COVID-19. The incubation period is the time from exposure to the virus to the development of symptoms or signs (positive testing) of the disease. Research indicates that the median incubation period is estimated to be 5.1 days, and 97.5% of those who develop symptoms will do so within 11.5 days of exposure. The term “median” refers to the number at which half the test population is less than that number, and have the test population is greater than that number. A median is often close to an average, but they are not the same and may differ significantly. These estimates imply that, under conservative assumptions, only about 100 out of every 10,000 cases will develop signs or symptoms after 14 days of exposure.
What tests are available for SARS-CoV-2?
COVID testing is now available and includes a panel of 3 tests:
- RT-qPCR test – Detects the presence of SARS-CoV-2 virus
- IgM serology test – Detects the early antibody response
- IgG serology test – Detects the mature antibody response
“Nucleic acid amplification tests,” or “NAAT”
The NAAT test is directed at identifying the presence of the ARS-CoV-2 virus. “Nucleic acid amplification tests,” or “NAAT” tests are molecular tests that detect the virus’s genetic material in a sample that typically comes from a patient’s respiratory system such as anasal or oral swab. FDA-authorized NAAT tests for SARS-CoV-2 meet the EUA statutory standard, and based on the current available data, the FDA believes they are highly accurate. This means that a positive or a negative result from a NAAT test is likely to be true.
RT qPCR (Reverse Transcription Quantitative Polymerase Chain Reaction)
RT-qPCR is a highly sensitive NAAT test for the SARS-CoV-2 virus. it has its limitations. RT-qPCR requires obtaining high-quality nasopharyngeal swabs containing sufficient amounts of viral RNA. The amount of viral RNA not only varies tremendously between patients, it can also vary within the same patient depending on the timing of the test regarding the start of the infection and the onset of symptoms. The RT-qPCR test sample is obtained by naso-pharyngeal or throat swab. Currently, due to the limited number of tests available in the country, RT-qPCR test is only obtained from patients with current symptoms suggests of SARS-CoV-2 illness.
While RT qPCR tests alone may not be sufficient to diagnose COVID-19, they can be a valuable diagnostic tool when combined with lgM/lgG serological (see below). lgM/lgG serological assays can be used in large-scale, whole-population, testing to assess the overall immune response to the virus and identify asymptomatic carriers of the virus. In fact, 20-80% of COVID-19 cases are estimated to be without symptoms.
A positive RT qPCR test indicates a person is carrying the virus and is likely contagious, regardless if they have symptoms or not.
Serology or Antibody Tests
In response to an infection, such as SARS-CoV-2, the body develops an overall immune response to fight the infection. One component of the immune system’s response is development of antibodies that attach to the virus and help eliminate it. A serology, or antibody test, measures the amount of antibodies present in the blood when the body is responds to a specific infection, like COVID-19. This means the test detects the body’s immune response to the infection caused by the virus rather than detecting the virus itself. Serological tests are recommended to be used on patients at least 3 days after onset of symptoms or 7-10 days after infection. The serology test sample is obtained by drawing blood.
IgM Antibody
The body’s initial immune reaction produces general antibodies that attack many infections, called “IgM” antibodies. IgM antibodies appear early and are mostly positive after 3-5 days of onset. A positive result for “IgM” antibodies is likely to indicate that someone currently is in the early phase of infection or has recently had the virus.
SARS-CoV-2 IgM antibodies then decrease while the SARS-CoV-2 IgG antibodies increase. During the recovery phase, the titer of the SARS-CoV-2 IgG antibody may increase four times or more compared to the acute phase.
Because it takes time for the body to make IgM antibodies in response to SARS-CoV-2, their absence does not mean that someone is not infected, particularly in those who have recently been in contact with the virus. A test for IgM antibodies may give a false negative result in a patient with SARS-CoV-2, particularly early in infection, even when they are symptomatic or asymptomatic but actively shedding the virus. Since IgM antibodies may not develop early or at all in infected patients, this type of antibody test is not used solely to rule out SARS-CoV-2 in an individual.
IgG Antibody
Over time, the body develops a second type of antibody in response to the infection that is more specific to the virus, called “IgG” antibodies. Most antibody tests detect IgG antibodies. On average, IgG antibodies take about 4 weeks to develop, but the time to development may vary substantially, and there is still a lot we do not know about SARS-COV-2. Since IgG antibodies generally do not develop until several weeks after infection, this type of antibody test, even though it is more specific to SARS-CoV-2, is not used solely to rule-out SARS-CoV-2 infection in an individual.
To Summarize:
(1) SARS-CoV-2 IgM antibodies appears early and are mostly positive after 3-5 days of onset.
(2) SARS-CoV-2 IgM titers then decrease while the SARS-CoV-2 IgG antibodies increase.
(3) During the recovery phase, the titer of the SARS-CoV-2 IgG antibody may increase four times or more compared to the acute phase.
False Negative Testing
As noted above, in the early days of an infection when the body’s immune response is still building, antibodies may not be detected, leading to a negative test result falsely indicating absence of disease. This limits the antibody test’s effectiveness for diagnosing COVID-19 and why it should not be used as the sole basis to diagnose COVID-19.
False Positive Testing
Positive results may also be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. None of these viruses are currently known to be causing active disease in this area at this time.
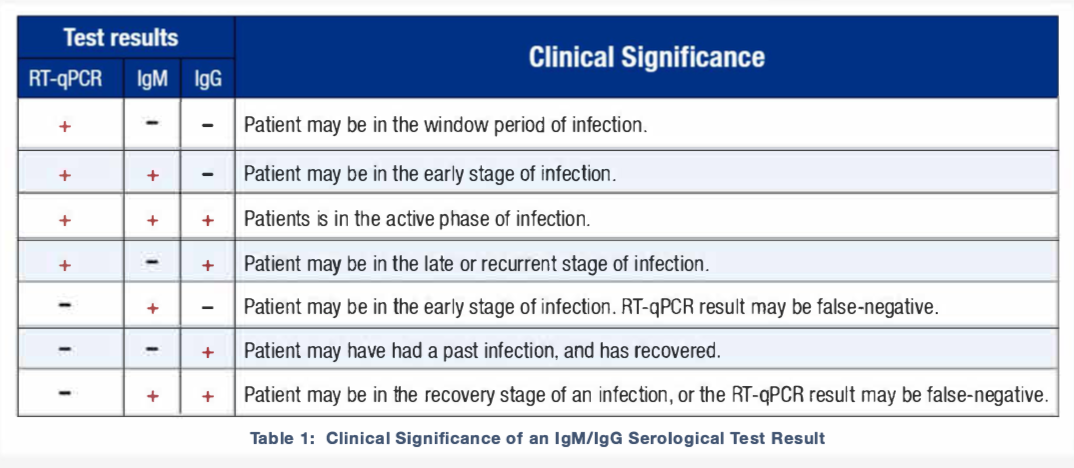
Interpretation of the 3 tests:
Does the presence of IgM/IgG antibodies mean one is immune to re-infection of SARS-CoV-2?
This is the million dollar question for which there, again, is no definitive answer. In general, the presence of significant levels of IgG antibodies confers relative immunity to the illness in question. By relative, again in general, it is meant that immunity is likely good for an indeterminate time but the strength of that immunity may fade over time which is why some vaccinations must be repeated every 5 or 10 years – for example, with tetanus vaccines.
In the case of SARS-CoV-2, there have been no studies that specifically confirm immunity against reinfection and some, including the World Health Organization, argue therefore that positive IgG testing should not be relied upon as confirming immunity. Which is likely good advice since there are few absolute certainties in this world. But the question then becomes, why shouldn’t it? To answer this, one needs to understand the basics of the immune response to illness such as SARS-CoV-2.
The development of immunity to a virus is a multi-step process that typically takes place over 1-3 weeks. First is a non-specific response by the body’s white cells (macrophages and neutrophils) that slow the progress of the virus and may prevent or reduce symptoms. This non-specific response is followed by a specific response where the body makes antibodies (such as IgM and IgG) that specifically bind to the virus, blocking its ability to cause disease. The body also makes T-cells that recognize and eliminate other cells infected with the virus. This T-cell response is called “cellular immunity” and it can be a very important and definitive component of developing immunity – such as the case of the HIV virus. This combined response by T-cells and antibodies ultimately clear the virus from the body and, if the response is strong enough, it may prevent re-infection by the virus.
It is not known to the extent that the T-cell response is necessary to convey future immunity to reinfection with SARS-CoV-2. There appears to be no research that argues that the presence of significant IgM/IgG levels DO NOT confer immunity, although again there is no consensus as to what specific levels are needed.
So, one may argue that while the presence of significant IgM/IgG levels may likely confer immunity, it is not absolute and should not be relied upon to assume absolute immunity to reinfection by SARS-CoV-2.
Conclusions
Overall, results of RT-qPCR and lgM/lgG serological tests do not necessarily need to agree. A disagreement between the two tests, if any, can often be traced to the time points at which the tests were performed. While RT-qPCR testing may be appropriate for the detection of the SARS-CoV-2 virus during the early, acute phase, lgM/lgG is an appropriate test during the chronic phase. Since the exact time of infection is often unknown, combining RT-qPCR and lgM/lgG testing can improve the accuracy of the COVID-19 diagnosis.
- A positive RT qPCR test indicates a person is carrying the virus and is likely contagious, regardless if they have symptoms or not.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have recently been in contact with the virus. Follow-up testing with a molecular diagnostic assay (RT-qPCR) should be considered to rule out infection in these individuals.
- Positive IgM or IgG antibody test results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- Results from IgM or IgG antibody testing should not be used as the sole basis to diagnose or exclude current SARS-CoV-2 infection or to inform reinfection status.
- When using both the SARS-CoV-2 IgM test and the SARS-CoV-2 IgG test, the clinical sensitivity is estimated at 89.89% (with a 95% confidence interval of 83.62% to 96.15%). This means that about 90% of the time the test is sensitive enough to identify the SARS-CoV-2 virus when it is present.
- When using both the SARS-CoV-2 IgM test and the SARS-CoV-2 IgG test, the clinical specificity is estimated at 96.50% (with a 95% confidence interval of 93.95% to 99.05%). This means that when positive, the test correctly identifies the specificvirus as SARS-CoV-2, and not another virus, 96% of the time.
What we do not know
- We do not know how long an asymptomatic patient testing positive for RT-qPCR remains contagious.
- We do not know how long IgM or IgG antibodies to SARS-CoV-2 will remain present in the body after the infection has been cleared.
- We also do not know whether or not the presence of IgM and/or IgG antibodies confirms immunity to re-infection. Nor is it known how long a person who has recovered from the virus may be at lower risk of infection if they are exposed to the virus again.
- With the other four coronaviruses currently endemic in humans, we know that immunity disappears gradually over time — it likely takes years but we don’t really know. Adults might get infected with the same virus they had as a child, but even if someone gets sick with the coronavirus again, the second infection might not be as bad.
Resources
References
COVID-19 Testing
COVID-19 Tests
COVID-19 Testing Information
- Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine – 2020
- Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). – 2020
- Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2 – an observational cohort study – 2020
- The lncubahon Period of Coronavirus Disease 2019 (COV:0- 19) From Publicly Reported Confirmed Cases – 2020
COVID-19 – Predictability of Serology Test Results
- “Immunity passports” in the context of COVID-19 – WHO 4-2020
- Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019
- The important role of serology for COVID-19 control – 4-2020
- Serology characteristics of SARS-CoV-2 infection since the exposure and post symptoms onset – 2020
- Evaluation of serological tests in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak – 2020
- Reinfection could not occur in SARS-CoV-2 infected rhesus macaques – 2020
Emphasis on Education
Accurate Clinic promotes patient education as the foundation of it’s medical care. In Dr. Ehlenberger’s integrative approach to patient care, including conventional and complementary and alternative medical (CAM) treatments, he may encourage or provide advice about the use of supplements. However, the specifics of choice of supplement, dosing and duration of treatment should be individualized through discussion with Dr. Ehlenberger. The following information and reference articles are presented to provide the reader with some of the latest research to facilitate evidence-based, informed decisions regarding the use of conventional as well as CAM treatments.
For medical-legal reasons, access to these links is limited to patients enrolled in an Accurate Clinic medical program.
Should you wish more information regarding any of the subjects listed – or not listed – here, please contact Dr. Ehlenberger. He has literally thousands of published articles to share on hundreds of topics associated with pain management, weight loss, nutrition, addiction recovery and emergency medicine. It would take years for you to read them, as it did him.
For more information, please contact Accurate Clinic.
Supplements recommended by Dr. Ehlenberger may be purchased commercially online or at Accurate Clinic.
Please read about our statement regarding the sale of products recommended by Dr. Ehlenberger.
Accurate Supplement Prices
.