 Marijuana (Cannabis)
Marijuana (Cannabis)
The Endocannabinoid System (ECS)
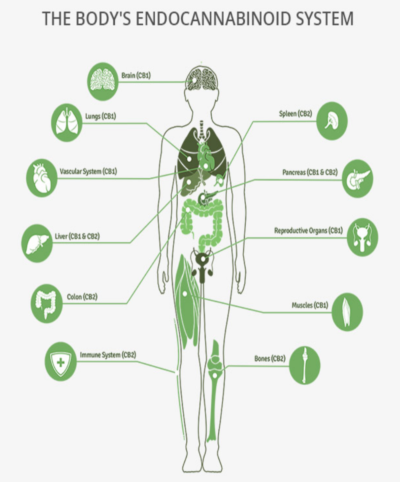
The endocannabinoid system (ECS) is a naturally occurring neuroendocrine network that is present throughout the body, including the brain, nervous system, heart and organs. The ECS regulates many physiologic functions including pain, inflammation, immunity, appetite and metabolism, gastrointestinal (GI) function, memory and movement. THC, CBD and other cannabinoids found in marijuana interact with the ECS to provide their therapeutic benefits.
Links to other Pertinent Educational Pages:
Cannabinoids:
- Cannabinoids
- Δ-9 THC
- Cannabidiol (CBD)
Terpenes:
Key to Links:
Grey text – handout
Red text – another page on this website
Blue text – Journal publication
The Endocannabinoid System
When research first began exploring how marijuana affects the human body, nothing was known about the system within the body that the constituents acted upon. The group of compounds found to be uniquely abundant in mariuana (cannabis) were compounds called “cannabinoids.” Originally thought to be unique to cannabis, naturally occuring cannabinoids were subsequently discovered in humans and animals (all vertebrates) which were named “endocannabinoids.” After this, an entire “endocannabinoid system” (ECS) was discovered, consisting of multiple types of endocannabinoids and cannabinoid receptors which were distributed throughout the body.
Function of the Endocannabinoid System (ECS)
The endocannabinoid system (ECS) regulates many physiologic functions ranging from the immune system to the nervous system and affects sleep, appetite, mood, pain and other functions. Moreover, the endocannabinoid system is involved in the modulation of other physiological functions, such as inflammation, endocrine function, cognition, memory, nausea, anti-nociception, and vomiting. Cannabinoids might also function to reduce stress and modulate cognitive and emotional functions.
The most understood functions of the ECS are related to regulation of the central nervous system (CNS) and immune function in the body. The ECS also plays a critical role in maintaining the skin and its barrier function. For example, dysregulation of the ECS has been implicated in various skin disorders like atopic dermatitis, itch, acne, hair growth/loss, and hyper/hypopigmentation.
Many pre-clinical animal and labatory-based studies have shown that modifying the activity of the ECS affects many medical conditions including: mood, anxiety disorders, movement disorders, neuropathic (nerve) pain, epilepsy, multiple sclerosis, spinal cord injury, cancer, atherosclerosis, myocardial infarction, stroke, hypertension, glaucoma, obesity/metabolic syndrome, insomnia, drug addiction, Alzheimer’s disease, and osteoporosis. However, there is a lack of good quality human-based research to confirm the specifics of what these pre-clinical studies suggest. It is been believed that some conditions including migraine headaches, fibromyalgia, and Irritable Bowel Syndrome (IBS) may be the result of an underlying endocannabinoid deficiency (see below), indicating they may be effectively treated with cannabinoid medications.
Endocannabinoids are produced in the post-synaptic membrane on demand in response to a stress event. They are produced by breakdown of phospholipids, a component of cell membranes and are not stored in vesicles like other neurotransmitters but released immediately. This results in a retrograde feedback loop to the pre-synaptic membrane to suppress neurotransmitter release.
The half-life of endocannabinoids is extremely short, with rapid breakdown by lipase enzymes and metabolism via liver cytochrome P450 (CYP) enzymes and subsequent biliary and intestinal excretion. The best known lipases are monoacyglycerol lipase (MAGL), which degrades 2-AG, and fatty acid amide hydrolase (FAAH) which predominantly degrades AEA. Each of these components is necessary for maintaining tight control over endocannabinoid levels and their actions on brain circuits.
Because cannabinoids are lipophilic (fat soluble), they may remain in fat tissues for a long time. Cannabinoid tolerance can occur at the receptor level via receptor internalization or degradation, reduced signalling or reduced protein synthesis.
Endocannabinoids are present in osteoarthritic and inflammatory arthritic joints, but not normal controls, suggesting synthesis following tissue injury or inflammation. This on-demand synthesis is analogous to the upregulation of the endogenous opioid system and endorphins in the setting of inflammation. Joints affected by inflammatory arthritis and osteoarthritis express cannabinoid receptors in the synovium and have increased endocannabinoids in the synovial fluid, in contrast to normal controls.
Endocannabinoids and Endorphins
The endocannabinoid system of naturally occurring endocannabinoids and receptors is similar to the opioid system of naturally occurring opioids (endorphins) and receptors in the body. The analgesic effect of cannabinoids as a result of binding to cannabinoid receptors has been confirmed, and the role of the endocannabinoid system in pain relief has been verified in various types of pain: somatic, visceral and neuropathy. Classical analgesics, nonsteroidal anti-inflammatory drugs, opioids, and antidepressants increase the activity of the endocannabinoid system.
Cannabinoids are not as effective as opioids in reducing acute pain but they appear to have increased effectiveness in chronic pain states.
Components of the ECS
The ECS is made up of multiple components:
- Signaling molecules called “cannabinoids”
- Specific receptors
- Enzymes that synthesize and breakdown endocannabinoids and transporters of endocannabinoids.
Cannabinoids
Cannabinoids can be broken into three general categories based on where they are produced:
- Endocannabinoids (ECBs): the cannabinoids compounds synthesized by the human body. The first endocannabinoid was identified in 1988, N-arachidonoylethanolamine (AEA), also known as anandamide, its name derived from the sanskrit word “ananda” which roughly translates to “bliss” or “joy.” Since then, other endocannabinoids have been reported but the most widely studied are AEA and 2-arachidonoyl glycerol (2-AG). Other less known endocannabinoids are N-palmitoyl ethanolamide (PEA), N-alpha-linolenoyl ethanolamide (ALEA) N-linoleoyl ethanolamide (LEA), N-oleoyl ethanolamide (OEA), N-stearoyl ethanolamide (SEA), N-eicosapentaenoyl ethanolamide (EPEA), and, N-docosahexaenoyl ethanolamide (DHEA).
- Phytocannabinoids (PCBs): the cannabinoids synthesized by plants. In cannabis, the most prominent phytocannabinoids are tetrahydrocannabinol (THC) and cannabidiol (CBD) but there are a number of other minor cannabinoids found in cannabis (See:Cannabinoids). While there are many cultivars (strains) of C. sativa, regulatory bodies typically segment them into one of two different chemotypes based on the chemical breakdown of the constituents found in the plant: Industrial hemp and Marijuana. Industrial hemp is the chemotype with a minimal amount of tetrahydrocannabinol (THC) and higher levels of CBD, while the marijuana chemotype contains high levels of THC (e.g., above 0.3% w/w by dry weight). Phytocannabinoids are found in abundance in the resin-producing trichomes of Cannabis sativa L. (C. sativa) and Cannabis Indica L (C Indica). See: Marijuana vs Hemp
- Synthetic cannabinoids (SCs): the cannabinoids manufactured synthetically using various chemical processes (e.g., the prescriptionvmedications dronabinol (Marinol) and nabilone (Cesamet).
Cannabinoid-Like Compounds
Palmitoylethanolamide (PEA)
Palmitoylethanolamide (PEA), an endogenous fatty acid amide, generates its neuromodulatory effects acting via several targets, including the GPR55 receptor although PEA may also indirectly activate CB1 and CB2 receptors. PEA has neuroprotective, anti-neuroinflammatory and analgesic properties and is used to treat nerve pain, intestinal inflammation and other conditions.
β-Caryophyllene
β-Caryophyllene (or caryophyllene), commonly found in significant amounts in marijuana and other plants, acts on the CB2 receptor and is therefore sometimes considered to be a cannabinoid. See: β-Caryophyllene
Curcuminoids
Curcuminoids are compounds found in turmeric that are thought to act on the CB1 receptor. See: Curcumin
Receptors
Cannabinoid receptors are present in the central nervous system and many peripheral tissues including spleen, white blood cells (leukocytes), reproductive, urinary and gastrointestinal tracts; endocrine glands, arteries, heart and skin. These receptors respond to the presence of cannabinoids in many differenst ways, whether the cannabinoid is naturally occurring in the body (endocannabinoids), plant-based (phytocannabinoids) or synthetically manufactured. The entire field of cannabinoid pharmacology remains in its infancy but is now offering many potential clinical applications.
The best known cannabinoid receptors are two G-protein-coupled receptors (GPCRs): cannabinoid receptors 1 (CB1) and 2 (CB2) and and G protein-coupled receptor 55 (GPR55). CB1 receptors are predominantly found in the central nervous system (CNS), whereas CB2 receptors are found mostly outside the CNS. The receptors CB1, CB2 and GPR55 are located extracellularly and intracellularly, in the mitochondria, Golgi apparatus and the nucleus. The receptors that are located on cell membranes are coupled with the G protein.
Endogenous activation of these receptors is dependent on the endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) respectively. Taken together, the actions of endocannabinoids depend both on expression of the target receptors on specific cells and on adaptations that are induced in different pain states within specific brain areas. Abundant preclinical data support that when these receptors are activated, pain stimulus is suppressed, reducing pain perception.
CB1 Receptors
CB1 receptors are predominantly found in the central nervous system (CNS), brain and spinal cord and are the most abundant GPCRs in the CNS where they have psychoactive effects. Mostly found in the CNS, CB1 receptors have the highest density in such structures as the hippocampus, cerebellum, basal ganglia, cerebral cortex, hypothalamus, dorsal vagus nerve complex, and spinal cord.
CB1 receptors are also found in the gastrointestinal (GI) system, fat cells, liver, skin and skeletal muscle. ∆9-THC, the most abundant phytocannabinoid found in marijuana, binds to the CB1 receptor as a CB1 receptor partial agonist. On the other hand, CBD is a negative allosteric modulator of the CB1 receptor. Curcuminoids found in turmeric are also thought to act on the CB1 receptor.
CB1 receptors moderate pain modulation, memory processing, motor function, and psychoactivity.
CB2 Receptors
In contrast, CB2 receptors are primarily found on immune cells located in the tonsils, thymus, spleen, and bone marrow. They are also found in the peripheral nervous system (nerves in extremities) and enteric nervous system (nerves in the GI tract). CB2 receptors are upregulated in inflammation. Historically, CB2 receptors were thought to be expressed exclusively in the periphery, primarily on immune cells including microglia, but evidence now shows that these receptors are also expressed in the central nervous system, including pain centers and descending pain pathways. CB2 receptors are found at lower concentrations compared to CB1 receptors in the midbrain and brainstem.
CBD, once thought to bind to the CB2 receptor, appears to have multiple mechanisms by which it exert its effects indirectly on both CB1 and CB2 receptors. The terpene, β-caryophyllene, commonly found in significant amounts in marijuana and other plants, acts on the CB2 receptor and is therefore sometimes considered to be a cannabinoid. Other phytocannabinoids known to act on the CB2 receptor are found in Echinacea and Magnolia Bark.
GPR55 Receptors
While CB1 and CB2 receptors are the best studied receptors in the ECS, both endocannabinoids and exogenous cannabinoids target other receptors. GPR55 is an orphan receptor that is stimulated by AEA and some lipophilic derivatives of the endocannabinoids. GPR55 is found on neurons in the dorsal root ganglion of the spinal cord (the site of pain processing), on adipose tissue and on microvascular endothelial cells suggesting a wide range of functions of the ECS that are still unknown.
A third receptor, the GPR55 receptor, has been identified as an atypical cannabinoid receptor and is implicated in multiple physiological processes although its functional role in the central nervous system is not fully understood. The presence of GPR55 receptor in neural regions such as the ventral hippocampus, which is critical for cognition, recognition memory and affective (mood) processing. This suggests that their activation may modulate mesolimbic activity states and related behavioral phenomena such as contextual memory and mood regulation.
Transient Receptor Potential channel TRPV1
Another binding site for AEA is the transient receptor potential channel TRPV1. AEA is a full agonist at TRPV1 channels found in pain circuits, including nociceptive primary afferents and many central neurons comprising ascending pain circuits. TRPV1 channels are stimulated by capsaicin, found in chili peppers and useful applied topically for pain. AEA is pro-nociceptive (increases pain signals) in some situations, promoting responses to painful stimuli but AEA activation of TRPV1 channels is also anti-nociceptive, especially in the presence of inflammation and neuropathic pain.
Endocannabinoids are synthesized primarily “on demand” in response to intense stimulation of afferents impinging on post-synaptic neurons that result in activation of postsynaptic metabotropic glutamate receptors. The endocannabinoids then travel retrogradely to the pre-synaptic neuron and inhibit the release of neurotransmitters from presynaptic terminals by binding to cannabinoid receptors on the presynaptic terminals. Cannabinoid agonists inhibit glutamate and GABA release in many areas of the brain.
The Endocannabinoid System – Skin
Research indicates that both CB1 and CB2 receptors are found in the skin, including epidermal keratinocytes (the primary cell of the epidermis which produce the keratin that makes up the outermost layer of skin), mast cells, melanocytes, eccrine glands (sebocytes) and cutaneous nerve fibers.
Keratinocytes
AEA controls the differentiation of keratinocytes. One known effect of CBD is lowering the activity of the FAAH enzyme responsible for degrading AEA; therefore, the ability of CBD to raise the AEA levels controlling skin differentiation may be one of the reasons for its effectiveness in skin products. CBD also induces antioxidant pathways in keratinocytes which may be helpful in atopic dermatitis. Early research suggests that activation of CB2 receptors topically with β-caryophyllene may enhance wound healing.
Mast Cells
Mast cells are carriers of histamine which underlies allergic reactions – but they are also involved in wound healing, immune responses, and angiogenesis (blood vessel formation). Mast cells respond to different types of injury by activating and regulating immune reactions by releasing several preformed and newly synthesized chemical mediators. Mast cells are important in modulating skin disorders including both acute and chronic inflammatory processes and pain (hyperalgesia). PEA modulates mast cells via a complex modulation of receptors and AEA causes them to release fewer proinflammatory molecules. Due to tight regulation of the skin by endocannabinoids, the activation of CB1 receptors show promise for treating skin allergies and other mast cell-dependent skin diseases.
Melanocytes
A third important skin cell type involved in the ECS are melanocytes, which produce the dark pigment melanin that protects the skin from UV radiation. Activation by AEA induces melatonin production (melanogenesis) in melanocytes. When skin is damaged by UV light, cannabinoid receptors play a role in modifying the damage. In melanoma cancer melanocytes divide uncontrollably and become deadly. Research suggests that activation of cannabinoid receptors in melanocytes may slow tumor growth and kill cancerous melanocyte cells.
Sebocytes
Sebocytes are highly specialized cells that produce the sebum (oil) that protects the outer layer of our skin. If these cells become overactive, the excess oil leads to acne. CBD, which suppresses sebocyte proliferation while also exerting anti-inflammatory effects offers potential as an acne treatment. Additionally, the transient receptor potential vanilloid-1 channels (TRPV1) regulate human sebocytes, and anandamide (AEA) activates these TRPV channels. Other phytocannabinoids from cannabis have shown effectiveness in the treatment for both dry skin and acne.
Cutaneous Nerve Fibers
Both CB1 and CB2 receptors are found on peripheral nerves including cutaneous nerve fibers found in the skin. Research indicates cannabinoids interacting with these receptors and the ECS appear to be effective in reducing nerve pain associated with diabetic and chemotherapy-induced peripheral neuropathies. It is also hypothesized that they may be effective for other painful peripheral nerve-based conditions such as neuromas and the allodynia and hyperalgesia associated with CRPS and other nerve injuries.
Research has identified topical THC, CBD, PEA and B-caryophyllene as providing early evidence for effectiveness in these clinical conditions. Other molecules including some terpenes such as linalool are also being evaluated for the treatment of these painful conditions.
Enzymes and Transporters
The body’s manufacturing of the endocannabinoid AEA is mediated by Phospholipase D while diacylglycerol lipase (DAGL) regulates the manufacturing of 2-AG. The degradation of AEA and 2-AG is primarily regulated by two enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively. The interaction of endocannabinoids with their receptors is inhibited by a two-steps: endocannabinoids are first removed from the intracellular space by a membrane transporter known as anandamide membrane transporter (AMT) and then, after reuptake, the endocannabinoids entering the cells are metabolized by enzymes like FAAH and MAGL
Fatty Acid Amide Hydrolase (FAAH)
Compounds that inhibit FAAH would be expected to enhance the effects of the endocannabinoid AEA by blocking its metabolism. Thus, FAAH inhibitors represent a potentially attractive therapeutic target for treatment of pain, inflammation, and other central nervous system (CNS) disorders. Known FAAH inhibitors include non-steroidal anti-inflammatory drugs (NSAIDs), CBD and palmitoylethanolamide (PEA).
Simplified scheme representing the pathogenesis of pain following inflammatory disease or nociceptive stimulus – from “Cannabinoid Delivery Systems for Pain and Inflammation Treatment” – Natascia Bruni
The Endocannabinoid System in Pain Modulation
Regarding the complex function of the endocannabinoid system in pain modulation, it is theorized that cannabinoids reduce the sensitization of nociceptive (pain) sensory pathways in chronic pain states. It is also theorized that a lack of endocannabinoids activity underlies the pathophysiology of fibromyalgia and other conditions (see below) but there is no clear evidence to support this theory yet. The endocannabinoids act as ligands at cannabinoid receptors CB1 and CB2; CB1 receptors are predominantly expressed in the central nervous system (CNS), whereas CB2 receptors are found mostly outside the CNS. Abundant preclinical data support that when these receptors are activated, pain stimulus is suppressed, influencing nociception.
Research indicates that drugs that selectively target CB1 receptors may not be clinically useful in some types of inflammatory and neuropathic pain. Conversely, cannabinoid agonists that bind to CB2 receptors may be beneficial for the treatment of inflammatory and neuropathic pain. CB2 receptors are also found in the central nervous system, including pain centers and descending pain pathways. Recent studies support the idea that CB2 receptors are relevant targets for chronic pain therapeutics for inflammatory and neuropathic pain.
The analgesic effects of cannabinoids and their ligands are primarily mediated by the CB1 receptor via inhibition of presynaptic gamma-aminobutyric acid (GABA) and glutamatergic transmission, which suppresses neuronal excitability. Although many cannabinoids have been identified, only THC and CBD (and the terpene/?cannabinoid β-caryophyllene) are known to be clinically relevant. THC and CBD act on CB1 and CB2 receptors while β-caryophyllene only acts on CB2 receptors.
THC is anti-inflammatory and influences pain, appetite, orientation, and mood; while CBD also has anti-inflammatory, anti-anxiety, and analgesic effects. Although THC and CBD both act on cannabinoid CB1 and CB2 receptors. THC is a receptor partial agonist, while CBD is a negative allosteric modulator of the CB1 receptor. Due to their individual properties, the proportion of THC to CBD in cannabis products impacts the therapeutic and adverse effects. Combining THC, CBD and β-caryophyllene creates a synergistic effect that provides benefit in using all three together for analgesia.
Tolerance
Plant-based cannabinoid receptor agonists, such as THC, excite cannabinoid receptors nonselectively, producing a nonphysiological response, because the body’s natural hydrolytic enzymes such as FAAH do not metabolize them. Thus, their effect lasts longer than natural endogenous cannabinoids (AEA and 2-AG) and cannot be controlled. The long-term effects of THC may reduce the amount of the CB1 receptor and, consequently, weaken the sensitivity of the endocannabinoid system resulting in tolerance to the THC. This tolerance and decreased effect may lead to a gradual increase in consumption frequency and/or dose. It is worth noting, however, that this tolerance is reversible in one to four weeks after discontinuation of THC use. There are nixed reports regarding tolerance, its time required for reversal and whether when reversed it can be reestablished quickly with repeat THC use.
That being stated, studies predominately find weak to no analgesic effects of cannabinoid agonists even though cannabinoids decrease functional connectivity of the “pain matrix” in functional magnetic resonance (fMRI) studies (Walter, et al., 2016). However, it should be noted that many of the fMRI studies have examined pain responses in healthy subjects to date, not subjects in chronic pain. The disconnect between pain relief in clinical studies and lack of reliable pain reduction produced by cannabinoids emphasizes the multifaceted aspects of pain and that analgesia is only one aspect of clinical pain relief. The modest effects of cannabinoids may be a result of effects of cannabinoids on sleep and mood. All studies conclude that more double-blind, placebo-controlled research is needed to understand the utility of cannabinoid therapies for pain.
An important strategy in pain management is to use ECS-related therapies in conjunction with other analgesics, such as NSAIDs or opioids. There is a substantial preclinical literature on synergistic analgesia produced by FAAH inhibitors, including CBD and palmitoylethanolamide (PEA), with morphine and other opioids for neuropathic pain. CB2 receptor agonists are also synergistic with morphine in animal models of acute and chronic inflammatory, post-operative, and neuropathic pain. β-Caryophyllene, a terpene with CB2 receptor agonist properties has growing research evidence for reducing pain. Studies suggest that lower opioid doses can be used effectively when used in combination with cannabinoid agonists along with decreased frequency of opioid-induced side-effects. Indeed, chronic pain patients reliably reduce their opioid consumption when using adjunct cannabis. However, long-term clinical use of cannabinoid therapies are at the early stage of investigation and more clinical trials are necessary to fully evaluate the efficacy of this class of drugs.
The Endocannabinoid System and the Descending Pain Modulatory System
The descending pain modulatory circuit is made up of the ventrolateral periaqueductal gray (PAG) projections to the rostral ventromedial medulla (RVM) and their reciprocal connections with upstream cortical and subcortical brain areas and downstream spinal cord neurons, respectively. Activation of the descending pain circuit typically results in analgesia. However, this circuit is subject to plasticity (change) during pain states, and prolonged pain results in a switch in the output from the RVM from inhibition of pain to facilitation of pain, indicating that the circuit is bi-directional in terms of pain modulation. Both the PAG and RVM integrate information from higher brain centers that integrate emotional and cognitive aspects of pain prior to regulating pain thresholds at the level of the spinal cord. Emotional aspects of pain are critical in the individual experience of chronic pain and thus the role of the ECS is important due to its impact on mood and emotion.
Medications that impact the descending pain modulatory circuit are frequently used for treating nerve pain including SNRO drugs (duloxetine (Cymbalta) and opioids.
The Endocannabinoid System and Inflammation
Cannabinoids are important in regulating inflammatory processes in the periphery. Many studies have shown that CB1 and CB2 receptor agonists, as well as FAAH and MAGL inhibitors, inhibit the development and maintenance of inflammation. Both CB1 and CB2 receptors inhibit edema and regulate the release of pro-inflammatory and anti-inflammatory cytokines. These peripheral effects of cannabinoids are important in the overall response to systemic administration of cannabinoid receptor agonists and other drugs that modulate the ECS in inflammation.
Clinical Endocannabinoid Deficiency (CED)
The ECS maintains an underlying endocannabinoid tone that reflects the levels of anandamide (AEA) and 2- arachidonoylglycerol (2-AG), the centrally acting endocannabinoids. This tone is balanced by their synthesis and breakdown as well as the relative density of cannabinoid receptors in the brain. If endocannabinoid function is decreased, a condition of Clinical Endocannabinoid Deficiency (CED), an associated lowered pain threshold would be present, along with disruption of digestion, mood, and sleep which are regulated by the ECS. The CED theory also proposes that such deficiencies could arise due to genetic or congenital reasons or be acquired due to injury or disease. As a consequence, characteristic pathological syndromes occur with particular symptoms.
The most evidence for CED is present for migraine, fibromyalgia, and irritable bowel syndrome (IBS). A strong argument can be made for unifying pathophysiological trends in these three conditions:
- Each of these conditions must be clinically diagnosed based on subjective criteria because they all lack characteristic tissue pathology or objective laboratory findings.
- Each of these conditions are diagnoses of exclusion that often require extensive negative diagnostic work-ups.
- Each of these conditions are and often labeled psychosomatic in origin or worse, wastebasket diagnoses, at one time or another by skeptical clinicians
- Each of these conditions are associated with hyperalgesia (excessive sensitivity to pain).
- Each of these conditions are generally associated with anxiety and depression.
- Comorbidity is often present in these three diagnoses. Primary headaches co-occur in up to 97% of fibromyalgia patients; up to 35% of chronic daily headache patients also fit clinical criteria of fibromyalgia; up to 32% of IBS patients also meet many criteria for fibromyalgia, while 32% of fibromyalgia patients also meet many criteria for IBS8.
- While some patients suffer from only one of these syndromes, there is increased lifetime risk for developing another or all three of these conditions.
Other disorders that may represent CED
Other disorders possibly related to CED rubric include: neonatal failure to thrive, cystic fibrosis, causalgia, brachial plexopathy, phantom limb pain, infantile colic, glaucoma, dysmenorrhea, hyperemesis gravidarum, repetitive miscarriages, post-traumatic stress disorder (PTSD), bipolar disorder and possibly many others.
Clinical Endocannabinoid Deficiency (CED) – Treatment
Various approaches for treating CED-related conditions are possible.
See: Getting Started
The simple, direct approach is to treat with potent CB1 agonists (Δ9-THC, Δ8-THC, Marinol, Nabilone). However, one needs to take into account that the ECS operates as a homeostatic regulator that may require only a gentle pharmacological nudge, rather than a forceful shove, by potent agonists. Thus, it is recommended to start treatment gently with curcumin due to its mild agonism of CB1 and with FAAH inhibitors (PEA or CBD) that will raise AEA levels. When using CBD, the use of additional synergistic constituents, including certain minor cannabinoids and terpenes is recommended.
When progressing to a more potent CB1 agonists, small doses of THC should be initiated which may jump-start the ECS and not induce tolerance. Although Δ8-THC (a less potent CB1 agonist compared to Δ9-THC), may provide therapeutic benefit, there is inadequate research regarding its safety and effectiveness to allow for its recommendation at this time. Since THC alone is often poorly tolerated by inexperienced patients, standardized whole cannabis extracts that contain additional synergistic and buffering constituents such as CBD and terpenes are preferable.
Beyond drug interventions, evidence suggests that lifestyle approaches should be integrated into the treatment of CED. Specifically, low-impact aerobic exercise has demonstrated beneficial effects on endocannabinoid function. Dietary supplentation with probiotics and prebiotics may help not only IBS symptoms but also the entire spectrum of CED conditions. Ultimately, a multimodal approach is most likely to be effective in treatment of CED.
Resources:
National Academy of Sciences
This website appears to be good resource for exploring medical marijuana.
References:
Endocannabinoid System (ECS) – Basic Science
- The Discovery of the Endocannabinoid System – 2012
- Endocannabinoid signaling at the periphery – 50 years after THC – 2015
- A Personal Retrospective – Elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs) – 2016
- Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review – 2015
- Cannabinoid Receptors – Nomenclature and Pharmacological Principles – 2013
- CB1 & CB2 Receptor Pharmacology – 2017
- GPR3 and GPR6, novel molecular targets for cannabidiol. – PubMed – NCBI – 2017
- The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents. – PubMed – NCBI – 2017
- Pharmacokinetics and pharmacodynamics of cannabinoids. – PubMed – NCBI
- Clinical Pharmacodynamics of Cannabinoids – 2004
- Affinity and Efficacy Studies of Tetrahydrocannabinolic Acid A at Cannabinoid Receptor Types One and Two. – 2017
- Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. – PubMed – NCBI
- Pharmacology of Cannabinoids
- Cannabinoid receptor 2 – Potential role in immunomodulation and neuroinflammation Review – 2013
- Cannabinoid Receptors and the Endocannabinoid System – Signaling and Function in the Central Nervous System – 2018
- Crystal Structure of the Human Cannabinoid Receptor CB1 – 2017
- The_Endogenous_Cannabinoid_System_A_Budding_Source
- Does Cannabis Composition Matter? Differential Effects of Delta-9-tetrahydrocannabinol and Cannabidiol on Human Cognition – 2017
- Evidence for THC versus CBD in cannabinoids – 2018
- Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review – 2015
- Cannabinoid Receptors – Nomenclature and Pharmacological Principles – 2013
- Care and Feeding of the Endocannabinoid System – A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System – 2014
- CB1 & CB2 Receptor Pharmacology – 2017
- Dissociation between morphine-induced spinal gliosis and analgesic tolerance by ultra-low-dose α2-adrenergic and cannabinoid CB1-receptor antagonists – 2018
- GPR3 and GPR6, novel molecular targets for cannabidiol. – PubMed – NCBI – 2017
- Role of the endocannabinoid system in diabetes and diabetic complications – 2016
- The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents. – PubMed – NCBI – 2017
- The Role of Endocannabinoids System in Fatty Liver Disease and Therapeutic Potentials – 2013
ECS – Diet
ECS – In Disease
- Role of the endocannabinoid system in diabetes and diabetic complications – 2016
- Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation – 2016
- The Role of Endocannabinoids System in Fatty Liver Disease and Therapeutic Potentials – 2013
- The endocannabinoid system in pain and inflammation – Its relevance to rheumatic disease – 2017
- The endocannabinoid system in pain and inflammation – Its relevance to rheumatic disease – 2018
- Endocannabinoid System: A Multi-Facet Therapeutic Target. – PubMed – NCBI
- Enhanced endocannabinoid tone as a potential target of pharmacotherapy. – PubMed – NCBI
- Targeting cannabinoid receptor CB2 in car
diovascular disorders- promises and controversies – 2012 - Targeting Cannabinoid Signaling in the Immune System – “High”-ly Exciting Questions, Possibilities, and Challenges – 2017
- Care and Feeding of the Endocannabinoid System – A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System – 2014
- Targeting cannabinoid receptor CB2 in cardiovascular disorders- promises and controversies – 2012
- The Endocannabinoid System and Heart Disease – The Role of Cannabinoid Receptor Type 2 – 2019
ECS – Pain
- Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation – 2016
- Dissociation between morphine-induced spinal gliosis and analgesic tolerance by ultra-low-dose α2-adrenergic and cannabinoid CB1-receptor antagonists – 2018
- Impact of Efficacy at the m -Opioid Receptor on Antinociceptive Effects of Combinations of m -Opioid Receptor Agonists and Cannabinoid Receptor Agonists – 2015
- Targeting CB2 receptors and the endocannabinoid system for the treatment of pain – 2009
- The Endocannabinoid System and Pain – 2009
- The endocannabinoid system in pain and inflammation – Its relevance to rheumatic disease – 2017
- The endocannabinoid system in pain and inflammation – Its relevance to rheumatic disease – 2018
- The Endocannabinoid System, Cannabinoids, and Pain – 2013
- Cannabinoid receptor 2 – Potential role in immunomodulation and neuroinflammation Review – 2013
- Cannabinoid CB2 Receptors Regulate Central Sensitization and Pain Responses Associated with Osteoarthritis of the Knee Joint
- Role of endocannabinoid system in dopamine signalling within the reward circuits affected by chronic pain – PubMed – 2019
- High Times for Painful Blues – The Endocannabinoid System in Pain-Depression Comorbidity – 2015
- Cannabinoid‐based therapy as a future for joint degeneration. Focus on the role of CB2 receptor in the arthritis progression and pain – an updated review – 2021
- The endocannabinoid-alcohol crosstalk – recent advances on a bi-faceted target – 2018
- Molecular Understanding of the Activation of CB1 and Blockade of TRPV1 Receptors – Implications for Novel Treatment Strategies in Osteoarthritis – 2018
- Dual-Acting Compounds Targeting Endocannabinoid and Endovanilloid Systems—A Novel Treatment Option for Chronic Pain Management – 2016
- The Role of the Brain’s Endocannabinoid System in Pain and Its Modulation by Stress – 2015
- Cannabinoids, the endocannabinoid system and pain- a review of preclinical studies – 2021
- The Endocannabinoid System as a Therapeutic Target in Diabetic Peripheral Neuropathic Pain- A Review – 2021
ECS – Entourage Effect (Synergy)
- A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. – PubMed – NCBI
- Cannabis and cannabis extracts – greater than the sum of their parts? – 2001
- Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol. – PubMed – NCBI – 2018
- Does Cannabis Composition Matter? Differential Effects of Delta-9-tetrahydrocannabinol and Cannabidiol on Human Cognition – 2017
- Evidence for THC versus CBD in cannabinoids – 2018
ECS – Palmitoylethanolamide (PEA)
ECS – Fatty Acid Amide Hydrolase (FAAH)
- Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders – 2009
- A Personal Retrospective – Elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs) – 2016
ECS – Related
- Cannabis-conclusions – 2017 National Academy of Sciences
- Cannabis-chapter-highlights – 2017 National Academy of Sciences
- Cannabis-report-highlights – 2017 National Academy of Sciences
- Clinical Endocannabinoid Deficiency (CECD): Can this Concept Explain Therapeutic Benefits of Cannabis in Migraine, Fibromyalgia, Irritable Bowel Syndrome and other Treatment-Resistant Conditions?-2004
- Cannabimimetic phytochemicals in the diet – an evolutionary link to food selection and metabolic stress adaptation? – 2016
- Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. – PubMed – NCBI
- Cannabinoids and Cytochrome P450 Interactions. – PubMed – NCBI Pharmacogenetics of Cannabinoids – 2018
- Adverse effects of medical can
nabinoids – a systematic review – 2008 - Cannabimimetic effects modulated by cholinergic compounds. – PubMed – NCBI
- Antagonism of marihuana effects by indomethacin in humans. – PubMed – NCBI
- Pharmacokinetics and pharmacodynamics of cannabinoids. – PubMed – NCBI
- Clinical Pharmacodynamics of Cannabinoids – 2004
- Affinity and Efficacy Studies of Tetrahydrocannabinolic Acid A at Cannabinoid Receptor Types One and Two. – 2017
- Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. – PubMed – NCBI
- Pharmacology of Cannabinoids
- Current-status-and-future-of-cannabis-research-Clin-Researcher-2015
Emphasis on Education
Accurate Clinic promotes patient education as the foundation of it’s medical care. In Dr. Ehlenberger’s integrative approach to patient care, including conventional and complementary and alternative medical (CAM) treatments, he may encourage or provide advice about the use of supplements. However, the specifics of choice of supplement, dosing and duration of treatment should be individualized through discussion with Dr. Ehlenberger. The following information and reference articles are presented to provide the reader with some of the latest research to facilitate evidence-based, informed decisions regarding the use of conventional as well as CAM treatments.
For medical-legal reasons, access to these links is limited to patients enrolled in an Accurate Clinic medical program.
Should you wish more information regarding any of the subjects listed – or not listed – here, please contact Dr. Ehlenberger. He has literally thousands of published articles to share on hundreds of topics associated with pain management, weight loss, nutrition, addiction recovery and emergency medicine. It would take years for you to read them, as it did him.
For more information, please contact Accurate Clinic.
Supplements recommended by Dr. Ehlenberger may be purchased commercially online or at Accurate Clinic.
Please read about our statement regarding the sale of products recommended by Dr. Ehlenberger.
Accurate Supplement Prices
.
