“Pain is not evil, unless it conquers us.”
– Charles Kingsley
Prescription Medications:
Tapentadol (Nucynta)
Tapentadol is a relatively new synthetic opioid medication used for the management of moderate to severe pain. It offers a different profile of benefits for managing pain than traditional opiates and therefore offers specific advantages based on the nature and character of the pain being treated.
It is recommended to first read the following sections to become familiarized with some of the terms and concepts related here:
Also see:
Definitions and Terms Related to Pain
Key to Links:
- Grey text – handout
- Red text – another page on this website
- Blue text – Journal publication
Opioids – Tapentadol (Nucynta)
Tapentadol is a unique opioid pain medication with features that make it a particularly desirable choice in the managment of a broad spectrum of acute and chronic pain conditions including post-surgical, musculoskeletal, and neuropathic pains. Tapentadol hydrochloride immediate release (IR) was approved by the FDA in 2008 for the treatment of moderate to severe acute pain and tapentadol extended release (ER) obtained FDA approval for moderate to severe chronic pain and neuropathic pain in 2011 and 2012, respectively.
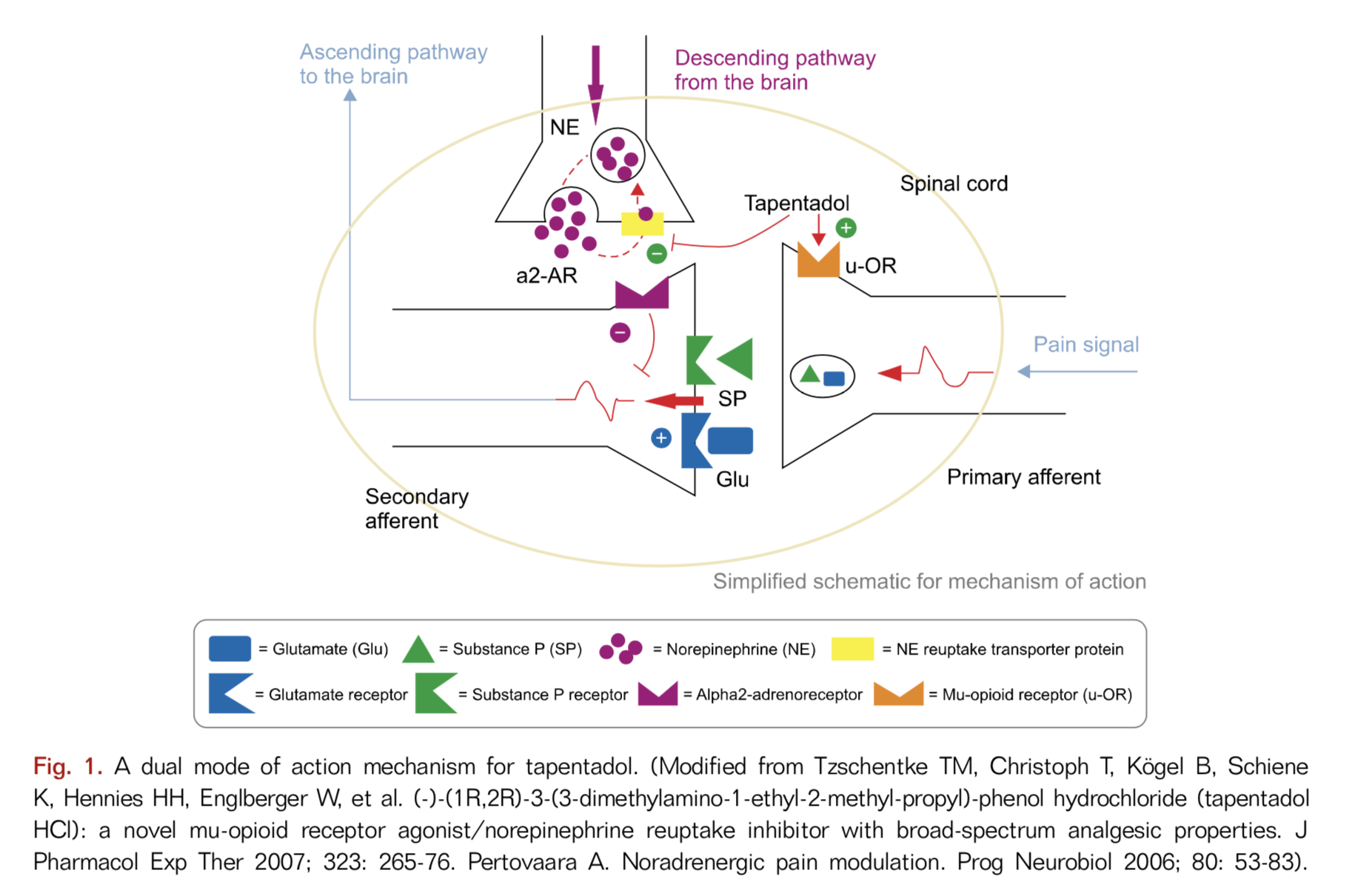
Tapentadol is different from traditional opioids due to its multiple mechanisms of action
Tapentadol represents a new class of centrally acting opioid analgesics with multiple mechanisms of action including a mu-opioid receptor agonist and a norepinephrine reuptake inhibitor (NRI). It has also been proposed to act as an alpha 2 agonist. In addition, tapentadol binds to the δ-opioid receptor (DOR) and κ-opioid receptor (KOR) although it is not certain how these actions contribute to tapentadol’s therapeutic effects or, possibly, its side effects.
Mu-opioid receptor agonist
Tapentadol functions by the same mechanism as “traditional” opioids such as morphine, hydrocodone (Norco), oxycodone and hydromorphone (Dilaudid). These opioids act primarily on mu-opioid receptors(MOR) in the brain and spinal cord to provide their pain-relieving benefits (and side effects). Much, but not all of ttapentadol’s pain relief comes from its MOR actity like these traditional opioids.
Norepinephrine reuptake inhibition (NRI) of the descending pathways
Tapentadol is also a norepinephrine reuptake inhibitor (NRI) which means that it enhances the pain inhibiting effects of the descending pathways (see Neurobiology of Pain) by enhancing the effects of the neurotransmitter norepinehphrine. The descending pathways are nerve pathways that descend from multiple areas in the brain down to the spinal cord that modify incoming pain signals to the brain. By modifying these incoming pain signals, the descending pathways alter the perception of the pain signals, resulting in either more or less pain. This secondary NRI function of tapentadol makes it more effective for nerve pain than usual opioids (see Neuropathic Pain). This NRI reuptake inhibition is also what makes duloxetine (Cymbalta) effective for pain.
Activation of alpha-2-adrenergic receptors
In addition, tapentadol provides additional pain suppression by activating alpha-2-adrenergic receptors in the spinal cord and brain. Studies have shown that naloxone, which blocks MORs, partially inhibits tapentadol-mediated analgesia. However, the addition of yohimbine which blocks alpha-2-receptors, to the naloxone eliminates all of tapentadol’s analgesic effects. This suggests that tapentadol’s activation of alpha-2-adrenergic receptors is also a signifcant contributor to tapentadol’s analgesic benefit.
See: Alpha-2 Adrenergic Agonists
Recently it has been hypothesized that tapentadol has yet another third important mechanism of action contributing to pain relief by inhibiting microglia activation. Microglia are immune cells found in the nervous system that are believed to play a major role in the evolution of acute pain to chronic pain by the release of active chemicals (cytokines) when activated by painful stimuli. Microglial activation is thought to contribute to the development of central sensitization, a condition associated with the chronification of pain. Central sensitization is a neuroinflammatory process characterized by a magnification of the perception of pain which leads to hyperalgesia, when painful stimuli are experienced to an exaggerated degree and allodynia, when non-painful stimuli are nevertheless experienced as painful.
Should this new hypothesis be valid, it argues strongly for tapentadol to be incorporated early in the opioid management of pain, perhaps as the first, or certainly one of the first, opioids to be engaged. Alternatively, it could be argued that tapentadol ER be prescribed for chronic pain as a long-acting agent to supplement or replace the use of short-acting opioids. This may be particularly relevant due to the growing evidence that traditional opioids contribute to microglial activation, neuroinflammation and central sensitization.
Tapentadol’s Clinical Effectiveness for Pain Conditions
Chronic Arthritis and Back Pain
Tapentadol is effective for moderate to severe pain related to chronic osteoarthritis-related hip and knee pain as well as chronic back pain with sciatica. A study of the effects of 300 mg per day of tapentadol ER in patients with severe chronic low back pain with sciatica demonstrated significant improvements in pain intensity, neuropathic pain-related symptoms, and quality of life.
An important study recently published in 2017 demonstrates an added benefit of adding palmitoylethanolamide (PEA) to tapentadol in the treatment of LBP. In this study it was shown that adding PEA 600 mg twice a day to tapentadol for LBP significantly enhanced pain control to a greater degree than tapentadol or PEA alone.
See: Palmitoylethanolamide (PEA)
Diabetic Peripheral Neuropathy (DPN)
Chronic pain due to Diabetic Peripheral Neuropathy (DPN) appears to be particularly responsive to tapentadol, perhaps more than traditional opioids. The only other medications approved for the treatment of DPN by the FDA are duloxetine (Cymbalta) and pregabalin (Lyrica).
Post-operative Pain
Tapentadol, which has proven benefit in the management of both acute and chronic pain, is also effective for treating post-operative pain. It may have an additional advantage over traditional opioids for treating post-operativel pain by reducing neuroinflammation through its action on the descending pain pathways and alpha-2 adrenoceptors. By reducing neuroinflammation, tapentadol may suppress the transition of acute to chronic post-opertive pain.
A recent study found that a single preemptive oral dose of tapentadol (75 mg) is effective in reducing perioperative analgesic requirements and acute postoperative pain, without added side effects. Additionally, combining tapentadol with palmitoylethanolamide (PEA), which also reduces neuroinflammation, may further suppress the transition of acute to chronic post-opertive pain..
Tapentadol’s Clinical Effectiveness Compared with Other Opioids
As described above, the analgesic effectiveness of tapentadol is only partially derived from opioid-mediated mechanisms. Recent research has demonstrated that only 40% of tapentadol’s analgesic benefit is derived from its action on the mu-opiod receptors (MORs). This explains its relatively lower frequency of side effects related to mu-opioid receptor agonism – see below. Importantly, this introduces a new concept in the assessment of opioid-based analgesics, “the mu-opioid load” of an analgesic.
Mu-Opioid Load
Traditionally, opioids have been described in terms of their “morphine equivalence (ME),” in which an individual opioid’s potency is compared to that of morphine in an effort to guide dosing for analgesia and provide insight as to an opioid’s potential for side effects, especially respiratory depression. While this concept has its usefulness, it is fraught with mis-application and misunderstanding due to a wide range of variability in the assignment of an ME value, since this value varies widely amongst individuals and even within the same individual at different times and different circumstances.
The principle behind the usefulness of the ME concept is that the therapeutic effects as well as the side effects of traditional opioids such as morphine and hydrocodone are strictly related to their activity at the mu-opioid receptor. Therefore, a traditional opioid’s mu-opioid load is essentially 100% and there is an expected correlation between the analgesic potency of an opioid with its propensity to cause respiratory depression, the most concerning side effect of an opioid. In. other words, the higher the ME of an opioid, the greater analgesic benefit is expected as well as greater risk for respiratory depression and accidental overdose.
The concept of morphine equivalence was popularized by CDC guidelines published in 2016 to guide physicians toward safer opioid prescribing habits and is based only on an opioid’s estimated analgesic benefit which is derived from clinical observations rather than any direct measure. Unfortunately, these guidelines have driven politically-based rather than science-based policies and recommendations with the assignation of ME as an end-all measure of appropriate prescribing.
This is most pertinent to the prescribing of tapentadol. Because of clinical observations that compare its effectiveness for pain with other opioids, especially oxycodone, tapentadol has been assigned by most guidelines as having an ME of 0.4, so that a 100 mg dose of tapentadol is considered equivalent to a 40 mg dose of morphine. As such, this computation is incorporated into regulatory and insurance policies based on mis-applied safety concerns. Because tapentadol’s mu-opioid load is actually only 0.4, its expected impact on mu-opioid related respiratory depression would only be 40% of its actual morphine analgesic equivalence (0.4) and therefore would be 0.16. In truth, this should be taken into consideration when prescribed within ME guidelines generated by institutions, regulatory agencies and insurance companies. But they are not.
Guidelines established by these organizations are based on safety concerns, particulary respiratory depression, and therefore should institute a more meaningful respiratory depression equivalence value. There has been some headway along these lines in that buprenorphine, once assigned an ME of 30-100, now has been discontinued from being assigned an ME when prescription guidelines are applied.
Research has supported this concept of mu-opioid load with tapentadol with the demonstration of reduced mu-opioid-related side effects. Ttapentadol has been shown to have less respiratory depression than morphine analgesic equivalent doses of oxycodone. For example, a 20 mg dose of oxycodone (30 ME) was determined to have significantly greater respiratory depressant effect than a 100 mg dose of tapentadol (40 ME). In addition, tapentadol has a strong safety record regarding overdose deaths. Recent research has confirmed that tapentadol-related overdose deaths have been limited in all, or almost all, cases to polypharmacy with alcohol, sedatives and other opioids.
Tapentadol compared with Oxycodone
Tapentadol IR (50-100 mg every 4-6 hours) has equal or superior potency to that of oxycodone IR (10 or 15 mg every 4-6 hours). Tapentadol IR therapy is generally well tolerated and is associated with significant less incidences of nausea, vomiting and constipation compared with oxycodone IR therapy. Tapentadol is generally considered to have an approximate analgesic morphine equivalency (ME) of 0.4. Given this ME, 50 mg of tapentadol provides the analgesic benefit equivalence of 20 mg of morphine or hydrocodone whereas 10mg of oxycodone, with a ME of 1.5, provides the analgesic benefit equivalence of 15 mg of morphine. Keep in mind, however that the concept of analgesic morphine equivalency has fundamental flaws which prohibits accuracy when applied to individuals, or even the same individual at different times.
A 2015 study compared the treatment of low back pain with neuropathic component (sciatica, or radicular pain down the leg(s). Doses of tapentadol ER 100-250 mg twice a day were compared with oxycodone ER 20-50 mg twice a day. Overall pain reduction was greater with tapentadol ER by 37% compared with oxycodone ER. Nerve pain reduction was also found to be superior with tapentadol ER compared with oxycodone ER.
Less Side Effects
Because tapentadol has a weak affinity for the mu-opioid receptor, there is a reduction in the common side effects associated with traditional opioid use: less nausea, less constipation, less tolerance and withdrawal and less tendency for abuse compared with oxycodone. The time required for patients to develop tolerance is longer with tapentadol than morphine: it has been shown that tolerance to morphine develops 2.5 times more rapidly than to tapentadol. A 2018 study identified no development of tolerance over 2 years of daily management with tapentadol.
As noted above regarding mu-opioid load and respiratory depression, tapentadol has been shown to have less respiratory depression than a morphine analgesic equivalent dose of oxycodone. In addition, studies have shown tapentadol ER (100–250 mg twice a day) demonstrates better gastrointestinal tolerability than did oxycodone controlled-release (Oxycontin): there is 40% less constipation with tapentadol ER compared with oxycodone ER. Apparently, tapentadol has still less constipation compared with tapentadol ER.
Tapentadol also appears to be more tolerable in patients who have previously failed treatment with more conventional opioid therapy, including transdermal buprenorphine, transdermal fentanyl and oral morphine. While side effects with tapentadol may be less frequent than with other opioids, all usual precautions should be taken toward avoiding typical opioid side effects with tapentadol.
Like buprenorphine, tapentadol has a lower impact on sex hormone concentrations than traditional, pure opioids like morphine and oxycodone and is therefore less likely to contribute to reduction in testosterone blood levels..
Tapentadol compared with Tramadol
Unlike the partial agonist tramadol which provides both serotonin and norepinephrine reuptake, the full agonist tapentadol provides norepinephrine reuptake but has relatively no activity to inhibit serotonin reuptake, making serotonin-related side effects such as nausea & vomiting less likely. Like tramadol, however, tapentadol is also a phenylpropylamine class of drug, making cross sensitivity between these two medications more likely. Tapentadol has a far less opioid receptor binding affinity (18 times less than morphine).
Also, tapentadol does not undergo cytochrome P450 metabolism whereas tramadol does, again making the argument for tapentadol to have less likelihood of drug-drug interactions. Furthermore, tramadol is a “prodrug” so in order for it to be effective, it must be metabolized by the cytochrome P450 system (Cyp2D6) into its pharmacologically active metabolite, O-desmethyltramadol. This makes tramadol susceptible to genetic variants that may reduce or increase Cyp2D6 activity. Up to 15% of European descendants have markedly reduced Cyp2D6 activity and are genetically unable to convert tramadol into its active metabolite, making tramadol ineffective for pain in this population.
Contraindications
Tapentadol is contraindicated in people with epilepsy or who are otherwise prone to seizures. It raises intracranial pressure so should not be used in people with acute head injuries, brain tumors, or other conditions which increase intracranial pressure. Like all opioids and other sedatives, at higher doses it increases the risk of respiratory depression so it should be used with caution in people with asthma, severe COPD/emphysema or untreated sleep apnea.
Dosing
Tapentadol (Immediate Release)
Recommended doses of tapentadol are 50 mg, 75 mg, or 100 mg every 4–6 hours on the basis of pain intensity. However, a second dose can be given 1 hour after the initial dose if it was found to be insufficient. The maximum daily dose is 600 mg.
Tapentadol ER
Tapentadol ER is supplied in tablets of varying doses of tapentadol hydrochloride at 58.24 mg, 116.48 mg, 174.72 mg, 232.96 mg, and 291.20 mg. These increments respectively correspond to 50 mg, 100 mg, 150 mg, 200 mg, and 250 mg of free tapentadol.
The ER form should initially be started at 50 mg every 12 hours with a maximum dose of 500 mg per day. The ER form is intended for twice a day dosing. This is intended for chronic pain relief and an IR form for acute pain. If patients have to be converted from tapentadol IR to tapentadol ER, the conversion between the two forms can be achieved quite simply; it is recommended that the patient receive half of the IR requirement on a daily basis as an ER form every 12 hours.
Tapentadol Taken With Other Medications
Tapentadol ER and Lyrica (pregabalin)
A 2014 study evaluating the treatment of low back pain with a neuropathic component compared a course of a 5o0 mg daily dose of Tapentadol ER with a course of 300 mg daily dose of Tapentadol ER coupled with a 300mg daily dose of pregabalin (Lyrica). The outcome revealed the two regimens to be equally effective in reducing pain symptoms, greater than placebo. The implications of this study are two fold: (1.) it suggests that a 500 mg dose of Tapentadol ER provides equal effectiveness for nerve pain as compared to using pregabalin and/or (2.) the use of pregabalin with Tapentadol ER allows for equivalent benefits at a lower, opioid-sparing dose.
Tapentadol ER and PEA (palmitoylethanolamide)
A study published in 2017 evaluated the benefit of tapentadol vs tapentadol plus PEA (palmitoylethanolamide) in the management of low back patients with a nerve pain component to their pain, including those with sciatic and burning or electric characteristics of their pain. The study revealed that while both arms of the study provided benefit, the addition of PEA provided greater benefit than tapentadol alone.
See: PEA (palmitoylethanolamide)
Tapentadol Metabolism and Drug interactions
Tapentadol does not require metabolic activation and does not have any active metabolites, making it is less susceptible to drug interactions and common genetic variants. In addition, tapentadol is not likely to affect the metabolism of other concurrently prescribed drugs.
Liver metabolism
Most drugs are broken down (metabolized) in the liver via oxidation by the cytochrome P450 system (Cyp450) or by glucuronidation. The Cyp450 system of enzymes is very susceptible to genetic variation as well as susceptible to being enhanced or suppressed by other medications. This makes medications that are metabolized primarily by Cyp450 enzymes more prone to individual variability in responsiveness, including effectiveness and side effects.
Tapentadol is primarily metabolized in the liver by glucuronidation via UGT2B7 and UGT1A9, a metabolic process not significantly influenced by genetic variants and other drug interactions. Only 13% of tapentadol is metabolized by Cyp450 (CYP2C19), making it less likely to be significantly affected by genetic variants or by the use of other medications that affect the Cyp450 system. Because tapentadol does not inhibit or induce Cyp450 enzymes, use of tapentadol is also unlikely to affect other medications metabolized by Cyp450. Overall, this means that tapentadol is less likely to cause significant drug interactions with other medications.
Other Drug Interactions
Of note, however, other drug interactions with tapentadol may occur that are potentially significant. As an opioid, it can cause impairment of judgement, drowsiness, suppression of breathing at high doses and it can increase serotonin levels in the brain leading to serotonin syndrome (See Serotonin Syndrome). Due to it’s NRI mechanism of action, it can also increase risk of seizures in people prone to seizures. Any other medications or drugs that share these side effects can magnify these symptoms when taken with tapentadol, requiring caution when using them with tapentadol.
Due to additive side effects as described above, caution should be used with combining tapentadol with other opioids, alcohol, antidepressants, MAO inhibitors, benzodiazepines, sleeping medications and any other sedative drugs or drugs that might drop blood pressure. If these other medications are to be taken with tapentadol, consider reducing usual doses and discuss their use with your physician.
Tapentadol and Seizures
Patients with a history of seizures may be at increased risk for seizures with tapentadol.
Abuse Potential of Tapentadol
Tapentadol has a relatively lower risk for abuse, diversion, and opioid doctor shopping than those of the other opioids, but possibly slightly higher than that of tramadol. Tapentadol IR abuse prevalence was lower than all other opioids except fentanyl IR. Tapentadol’s abuse risk for tapentadol ER is lowest compared to fentanyl ER, tramadol ER, morphine ER, oxycodone ER, and oxymorphone ER, with the exception of hydromorphone ER. The endorsement of recreational abusers for tapentadol is lower than that of oxymorphone, but slightly high- er than that of tramadol.
Neurobiology of Tapentadol
Pharmacodynamics
Tapentadol is centrally-acting and is 18 times less potent than morphine in binding to the human mu-opioid receptor and is 2-3 times less potent in producing analgesia, with a morphine equivalence value of 0.4 in analgesic effect. Tapentadol inhibits norepinephrine reuptake resulting in increased norepinephrine concentrations and alpha-2 adrenoceptors activation. The analgesic activity due to the mu-opioid receptor agonist activity of tapentadol can be antagonized by selective mu-opioid antagonists (e.g., naloxone), whereas the norepinephrine reuptake inhibition is sensitive to norepinephrine modulators. Tapentadol has no pharmacologically active metabolites.
Intrinsic activity: The intrinsic activity of tapentadol at the μ receptor is relatively high, with a greater receptor reserve than that of oxycodone and morphine, potentially explaining the fewer side effects related to μ receptors and less development of tolerance.
Effects on the cardiovascular system: Tapentadol has no effect on the QT interval or other EKG parameters (heart rate, PR interval, QRS duration, T-wave or U-wave morphology).
Pharmacokinetics
Absorption: Bioavailability after single-dose administration (fasting) is approximately 32% due to extensive first-pass metabolism. Maximum serum concentrations of tapentadol are typically observed at around 1.25 hours after dosing. Dose-proportional increases in the Cmax and AUC values of tapentadol occur over the 50 to 150 mg dose range.
Food Effect: The AUC and Cmax increase by 25% and 16%, respectively, when tapentadol is administered after a high-fat, high-calorie breakfast. Tapentadol may be given with or without food.
Distribution: Tapentadol is widely distributed throughout the body. The plasma protein binding is low and amounts to approximately 20%.
Metabolism and Elimination: The metabolism of tapentadol is extensive with about 97% of the parent compound metabolized. Tapentadol is mainly metabolized in the liver via Phase 2 pathways, glucoronidation, and only a small amount is metabolized by Phase 1 oxidative pathways. The major pathway of tapentadol metabolism is conjugation with glucuronic acid to produce pharmacologically inactive glucuronides. After oral administration approximately 70% sulfate of tapentadol) of the dose is excreted in urine in the conjugated form.
A total of 3% of drug is excreted in urine as unchanged drug. Tapentadol is additionally metabolized to N-desmethyl tapentadol (13%) by CYP2C9 and CYP2C19 and to hydroxy tapentadol (2%) by CYP2D6, which are further metabolized by conjugation. None of the metabolites contributes to the analgesic activity.
Kidney Disease/Impaired Function
Because tapentadol is metabolized in the liver with no active metabolites and only 3% of a dose is excreted by the kidney, so tapentadol is generally safe in those with renal impairment. However, the safety and effectiveness of tapentadol has not been established in patients with severe renal impairment and it is not recommended in this population.
Liver Disease/Impaired Function
Administration of tapentadol in patients with impaired liver function results in higher blood levels compared to patients with normal liver function. Therefore, caution should be used with tapentadol in patients with liver disease and avoided in those with severe liver disease.
Drug Interactions
Tapentadol is mainly metabolized by Phase 2 glucuronidation, a high capacity/low affinity system, therefore, clinically relevant interactions caused by Phase 2 metabolism are unlikely to occur. Tapentadol neither either inhibits or induces Phase 1 cytochrome P450 enzymes so clinically relevant interactions mediated by the cytochrome P450 system are also unlikely to occur.
The pharmacokinetics of tapentadol are not affected when gastric pH or gastrointestinal motility are increased by omeprazole and metoclopramide, respectively. Plasma protein binding of tapentadol is low (approximately 20%). Therefore, the likelihood of pharmacokinetic drug-drug interactions by displacement from the protein binding site is also low.
References
Tapentadol – New Publications recently uploaded
- Patient considerations in the use of tapentadol for moderate to severe pain – 2013
- Opioid Safety and Concomitant Benzodiazepine Use in End-Stage Renal Disease Patients – 2019
- The mu-opioid receptor agonist:noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia- the case of tapentadol – 2014
- Pharmacological rationale for tapentadol therapy – a review of new evidence First evaluation of tapentadol oral solution for the treatment of moderate to severe acute pain in children aged 6 to <18 – 2019
- Tapentadol vs oxycodone:naloxone in the management of pain after total hip arthroplasty in the fast track setting – an observational study – 2019
- Evaluation of Abuse and Route of Administration of Extended-Release Tapentadol Among Treatment-Seeking Individuals – 2020
- Tapentadol Prolonged Release for Severe Chronic Osteoarthritis Pain in the Elderly—A Subgroup Analysis of Routine Clinical Practice Data – 2020
- Lasting Prolonged-Release Tapentadol for Moderate:Severe Non-Cancer Musculoskeletal Chronic Pain – 2015
- Neural Circuits: An investigation into the noradrenergic and serotonergic contributions of diffuse noxious inhibitory controls in a monoiodoacetate model of osteoarthritis – 2019
- Tapentadol Prolonged Release – A Review in Pain Management – 2018
- Repeated Administration of Clinical Doses of Tramadol and Tapentadol Causes Hepato- and Nephrotoxic Effects in Wistar Rats – 2020
- A combination pharmacotherapy of tapentadol and pregabalin to tackle centrally driven osteoarthritis pain – 2019
- Single dose analgesic efficacy of tapentadol in postsurgical dental pain- the results of a randomized, double-blind, placebo-controlled study – 2018
- Current considerations for the treatment of severe chronic pain- the potential for tapentadol – 2012
- Patient and Disease Characteristics Associate With Sensory Testing Results in Chronic Pancreatitis – 2019
- Efficacy and Safety of Long-Term Administration of Tapentadol in Relieving Chronic Pancreatitis Pain – PubMed 2017
- Efficacy and Safety of Long-Term Administration of Tapentadol in Relieving Chronic Pancreatitis Pain – 2017
- Miotic and Subject-Rated Effects of Therapeutic Doses of Tapentadol, Tramadol and Hydromorphone in Occasional Opioid Users – 2014
- Tapentadol treatment results in long-term pain relief in patients with chronic low back pain and associates with reduced segmental sensitization – 2020
- Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. – 2014
- Safe Use of Opioids in Chronic Kidney Disease and Hemodialysis Patients – Tips and Tricks for Non-Pain Specialists – 2020
- Considering tapentadol as a first-line analgesic 14 questions – PubMed – 2017
- Tapentadol vs oxycodone:naloxone in the management of pain after total hip arthroplasty in the fast track setting – an observational study – 2019
- Tapentadol is effective in the management of moderate-to-severe cancer-related pain in opioid-naïve and opioid-tolerant patients – a retrospective study – 2020
- Practical Considerations for the Use of Tapentadol Prolonged Release for the Management of Severe Chronic Pain – 2015
- Tapentadol inhibits calcitonin gene-related peptide release from rat brainstem in vitro – PubMed
- The analgesic agent tapentadol inhibits calcitonin gene-related peptide release from isolated rat brainstem via a serotonergic mechanism – PubMed
- Tapentadol Prolonged Release – A Review in Pain Management – 2018
- Pharmacological rationale for tapentadol therapy – a review of new evidence – 2019
- Evaluation of Abuse and Route of Administration of Extended- Release Tapentadol Among Treatment-Seeking Individuals, as Captured by the Addiction Severity Index–Multimedia Version (ASI-MV) – 2020
- Tapentadol Prolonged Release for Severe Chronic Osteoarthritis Pain in the Elderly—A Subgroup Analysis of Routine Clinical Practice Data – 2020
- Tapentadol Extended Release in the Treatment of Severe Chronic Low Back Pain and Osteoarthritis Pain – 2018
- Descending Noradrenergic Inhibition An Important Mechanism of Gabapentin Analgesia in Neuropathic Pain – PubMed – 2018
- Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System – 2019
Tapentadol – Overviews
- Nucynta prescribing info
- Tapentadol immediate release: a review of its use in the treatment of moderate to severe acute pain. – 2010 PubMed – NCBI
- Tapentadol hydrochloride – A novel analgesic – 2013
- Patient considerations in the use of tapentadol for moderate to severe pain – 2013
- Tapentadol – a Novel μ-Opioid Receptor Agonist:Norepinephrine Reuptake Inhibitor with Broad Spectrum Analgesic Properties – 2007
- Serotonin-Norepinephrine Reuptake Inhibitors for Pain Control – Premise and Promise – 2009
- Practical Considerations for the Use of Tapentadol Prolonged Release for the Management of Severe Chronic Pain – 2014
- Tolerability, Safety, and Quality of Life with Tapentadol Prolonged Release (PR) Compared with Oxycodone:Naloxone PR in Patients with Severe Chronic Low Back Pain with a Neuropathic Component – 2015
- Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, doub… – PubMed – NCBI
- The switch from buprenorphine to tapentadol – is it worth? – 2016
- Tapentadol_Can_It_Kill_Two_Birds_with_One_Stone_without breaking windows? – 2016
- Tapentadol for neuropathic pain – a review of clinical studies – 2019
- Pharmacological rationale for tapentadol therapy – a review of new evidence – 2019
- Tapentadol Prolonged Release – A Review in Pain Management – 2018
- Long-Term Effectiveness and Tolerability of Pain Treatment with Tapentadol Prolonged Release – 2021
Tapentadol – Cancer Pain
- Tapentadol prolonged release for severe chronic cancer-related pain – Effectiveness, tolerability, and influence on quality of life of the patients – 2015
- Ready Conversion of Patients with Well- Controlled, Moderate to Severe, Chronic Malignant Tumor–related Pain on Other Opioids to Tapentadol Extended Release – 2014
- Tapentadol – a new option for the treatment of cancer and noncancer pains – 2019
- Tapentadol in the management of cancer pain – current evidence and future perspectives – 2019
Tapentadol – Knee Pain
- Efficacy and Safety of tapentadol extended release compared with oxycodone controlled release.
- Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, doub… – PubMed – NCBI
Tapentadol – Low Back Pain
- Tapentadol Prolonged Release Versus Strong Opioids for Severe, Chronic Low Back Pain – 2013
- Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, doub… – PubMed – NCBI
- Tapentadol – an effective option for the treatment of back pain – 2019
Tapentadol – Neuropathic Pain
Tapentadol, Neuropathic Pain – Descending Pathways
- Descending Noradrenergic Inhibition – An Important Mechanism of Gabapentin Analgesia in Neuropathic Pain – 2018
- Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System – 2019
- Rescue of Noradrenergic System as a Novel Pharmacological Strategy in the Treatment of Chronic Pain – Focus on Microglia Activation – 2019
Tapentadol, Neuropathic Pain – Diabetic Peripheral Neuropathy
- Tapentadol extended release in the management of peripheral diabetic neuropathic pain – 2015
- Enhancement of antinociceptive effect of morphine by antidepressants in diabetic neuropathic pain model – 2014
- Comparison of Amitriptyline, Duloxetine, and Pregabalin in DPN – 2012
- Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy – 2014
Tapentadol, Neuropathic Pain – Low Back Pain & Sciatica
- Efficacy of Duloxetine in Chronic Low Back Pain with a Neuropathic Component – A Randomized, Double-blind, Placebo-controlled Crossover Trial – 2015
- Effectiveness and Safety of Tapentadol Prolonged Release (PR) Versus a Combination of Tapentadol PR and Pregabalin for the Management of Severe, Chronic Low Back Pain With a Neuropathic Component – 2014
- The beneficial use of ultramicronized palmitoylethanolamide as add-on therapy to Tapentadol in the treatment of low back pain – a pilot study – 2017
- Tapentadol – an effective option for the treatment of back pain – 2019
Tapentadol, Neuropathic Pain – Postoperative Pain
- Role of preemptive tapentadol in reduction of postoperative analgesic requirements after laparoscopic cholecystectomy – 2016
- Single-dose-analgesic-efficacy-of-tapentadol-in-postsurgical-dental-pain-the-results-of-a-randomized-double-blind-placebo-controlled-study-2018
Tapentadol – Safety
- Efficacy and Safety of Tapentadol Immediate Release Assessment in Treatment of Moderate to Severe Pain- A Systematic Review and Meta-Analysis – 2017
- Review of Post-Marketing Safety Data on Tapentadol, a Centrally Acting Analgesic – 2017
Tapentadol – Taken With Other Medications
- The beneficial use of ultramicronized palmitoylethanolamide as add-on therapy to Tapentadol in the treatment of low back pain – a pilot study – 2017
- Effectiveness and Safety of Tapentadol Prolonged Release (PR) Versus a Combination of Tapentadol PR and Pregabalin for the Management of Severe, Chronic Low Back Pain With a Neuropathic Component – 2014
Tapentadol – Compared to Other Medications
Tapentadol compared – Morphine Equivalence
- Does ‘Strong Analgesic’ Equal ‘Strong Opioid’? Tapentadol and the Concept of ‘µ-Load’ – 2018.pdf
- Safety concerns with the Centers for Disease Control opioid calculator – 2018
- Opioid-Morphine-EQ-Conversion-Factors-April-2017
Tapentadol compared – Oxycodone
- Tapentadol immediate release: a review of its use in the treatment of moderate to severe acute pain. – 2010 PubMed – NCBI
- Tolerability, Safety, and Quality of Life with Tapentadol Prolonged Release (PR) Compared with Oxycodone:Naloxone PR in Patients with Severe Chronic Low Back Pain with a Neuropathic Component – 2015
- A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain
- A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain
- An Experimental Study Comparing the Respiratory Effects of Tapentadol and Oxycodone in Healthy Volunteers – 2017
Tapentadol compared – Antidepressants and Lyrica
- Comparison of Amitriptyline, Duloxetine, and Pregabalin in DPN – 2012
- Effectiveness and Safety of Tapentadol Prolonged Release (PR) Versus a Combination of Tapentadol PR and Pregabalin for the Management of Severe, Chronic Low Back Pain With a Neuropathic Component – 2014
- Rescue of Noradrenergic System as a Novel Pharmacological Strategy in the Treatment of Chronic Pain – Focus on Microglia Activation – 2019
Emphasis on Education
Accurate Clinic promotes patient education as the foundation of it’s medical care. In Dr. Ehlenberger’s integrative approach to patient care, including conventional and complementary and alternative medical (CAM) treatments, he may encourage or provide advice about the use of supplements. However, the specifics of choice of supplement, dosing and duration of treatment should be individualized through discussion with Dr. Ehlenberger. The following information and reference articles are presented to provide the reader with some of the latest research to facilitate evidence-based, informed decisions regarding the use of conventional as well as CAM treatments.
For medical-legal reasons, access to these links is limited to patients enrolled in an Accurate Clinic medical program.
Should you wish more information regarding any of the subjects listed – or not listed – here, please contact Dr. Ehlenberger. He has literally thousands of published articles to share on hundreds of topics associated with pain management, weight loss, nutrition, addiction recovery and emergency medicine. It would take years for you to read them, as it did him.
For more information, please contact Accurate Clinic.
Supplements recommended by Dr. Ehlenberger may be purchased commercially online or at Accurate Clinic.
Please read about our statement regarding the sale of products recommended by Dr. Ehlenberger.
Accurate Supplement Prices