Enormous possibilities for health and creativity are held captive by your likes and dislikes. Inspecting your desires and attachments to food and making choices intuitively with discrimination will make your spiritual practice and every other relationship more rewarding. – Leonard Perlmutter
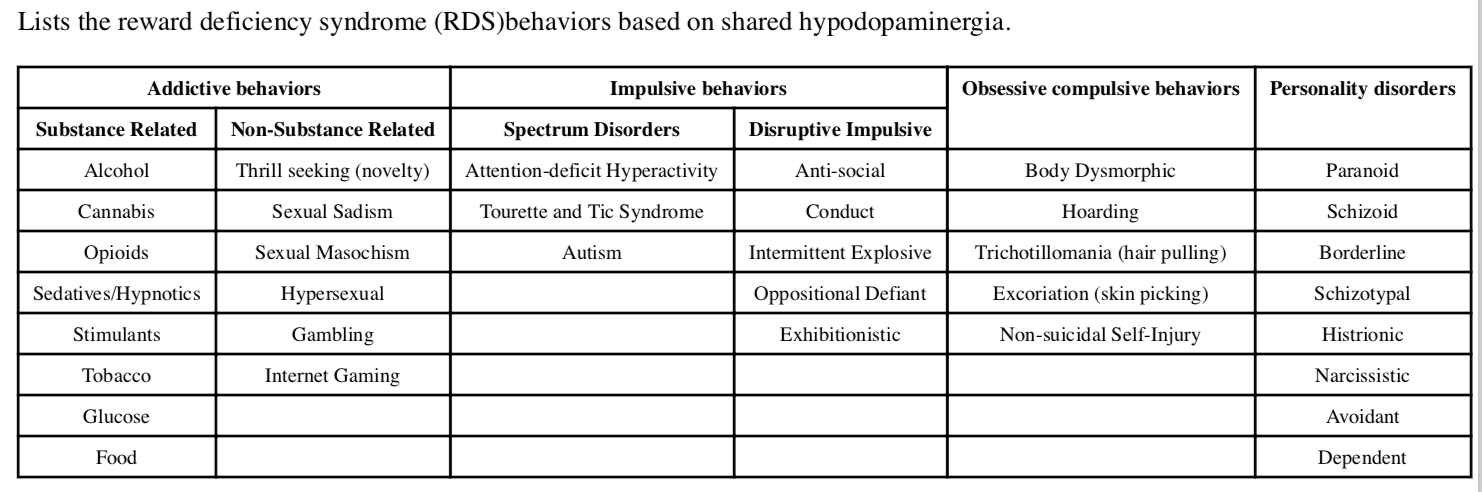
Reward Deficiency Syndrome (RDS) & Addiction
The common denominator underlying both chemical and behavioral addictions that also contributes to compulsive disorders such a obsessive compulsive disorder (OCD) as well as eating disorders, PTSD, ADD and certain anger disorders. It also overlaps with and influences chronic pain.
“Reward Deficiency Syndrome,” a term first coined by Ken Blum in 1995, can be defined as:
“A brain reward genetic dissatisfaction or impairment that results in aberrant pleasure seeking behavior that includes drugs, excessive food, sex, gaming/gambling and other behaviors.”
These other behaviors include an array of disorders, such as PTSD, ADHD, Tics, Tourette Syndrome, autism, Asperger Syndrome, OCD, “compulsive” sexual practices, binge eating and others. The relationships of these disorders becomes apparent with the understanding of the common genetic factors underlying them.
See:
Genetic Testing: Reward Deficiency Syndrome
See also:
AA/NA and 12-Step Programs
Addiction Recovery
Complementary & Alternative Addiction Recovery
Dopamine Diet
Dopamine Enhancement
Definitions and Terms Related to Pain
Key to Links:
Grey text – handout
Red text – another page on this website
Blue text – Journal publication
.
Reward Deficiency Syndrome (RDS) & Addiction
See first: Reward Deficiency Syndrome (RDS)
The Underlying Basis for Drug Addiction
Why do some people become addicted to drugs, while others do not?
Initially, a person may use substances for several reasons that seem to be positive effects, such as to experience the euphoric effects, to relieve stress, to overcome anxiety or depression (or both), or to blunt pain, physical or psychological. A person may continue a number of behaviors, such as gambling, sex, computer gaming or other activities for the same motivations. They also may believe that they can control their substance use or behavior.
But with repeated exposure, however, substance use or behaviors in some people can become uncontrollable and the person begins to lose their ability to stop the behavior or substance use, even when continuing to do so becomes harmful or disruptive to their lives. When away from the substance or behavior they develop compulsive desires and cravings to resume the behavior or substance use. This compulsive desire leads to loss of control which are the defining characteristics of addiction.
The evolution of understanding addiction as a character flaw or failure of will power to realizing that addiction is a chronic brain disease related to “reward deficiency” provides a major paradigm shift in the approach to treatment towards a neurobiologic-based approach. The heart of understanding the neurobiology of addiction is to understand the neurophysiologic correlates of perceiving the reward of feeling good, experiencing pleasure and the sense of well-being.
The Neurobiology of Reward and Addiction
The addictive process represents a chronic disease that is manifested by changes in the brain that are influenced by genetic and environmental factors. Brain imaging studies from drug-addicted subjects show physical changes in areas of the brain that are critical to judgment, decision-making, learning, memory and behavior control. The essence of addiction revolves around a reward response, in which behaviors and chemicals trigger a sense of reward associated with pleasure and perception of well-being. The understanding of reward systems and behavior begins with recognizing the basic drives that sustain life and survival of both the individual and the species, including eating and sexual behavior.
The primary rewarding effects of addictive substances and behaviors occur in areas of the brain that link basic emotions and connect them to memories, which drive behavior. These behaviors produce sensations of pleasure in response to actions that support survival (e.g., eating, sex) but also sensations of fear in response to potential dangers contributing to stress. These sensations in turn trigger the endocrine (hormone) and autonomic nervous systems, stimulating bodily responses. The brain’s reward and stress functions can be seen to reinforce life-sustaining behaviors.
Areas of the brain involved in the perception of reward, pleasure and well-being are referred to as the reward system of the brain, functionally inter-connected by different neural circuits or reward pathways. Neurotransmitters are chemical messengers that bind to specific receptors on nerve cells that allows communication between nerves cells making up neural pathways and dopamine (DA) is the most important neurotransmitter involved in the reward system, directing both pleasure and stress reduction.
While activation of this dopamine-based (dopaminergic) reward system results in feelings of reward and pleasure, reduced activity (hypo-dopaminergic functioning) results in a lack of pleasure and sense of well-being that can trigger dopamine-enhancing behavior such as drug-seeking or gambling. Mechanisms of hypo-dopaminergic functioning include reduced DA receptor density, blunted response to DA, or increased DA breakdown in the reward pathways. Many of these mechanisms leading to hypo-dopaminergic functioning can be induced by genetic variants which is why heredity plays a significant role in addiction risk. Stopping chronic drug use also generates a hypo-dopaminergic state that can trigger compulsive drug-seeking behaviors in an attempt to reverse the undesirable withdrawal-induced state.
Most addictions, including alcohol, opiates, psycho-stimulants (cocaine, methamphetamine), nicotine, glucose, gambling, sex addiction, excessive spending, and even uncontrolled internet gaming stimulate the release of DA in the reward pathways of the brain.
Importantly, the use of most addictive substances increases the levels of dopamine in the brain reward centers well beyond what occurs in naturally rewarding situations (e.g., sex, food). While the mechanisms are different for each drug, the excessive dopamine disrupts normal nerve signaling in the brain. The brain adjusts to excess dopamine levels by producing less dopamine and by reducing the number of receptors that respond to it in the receiving (postsynaptic) neuron. As a result, the pleasurable effects of a drug become diminished with continued use. Worse, because the number of DA receptors is reduced, the pleasurable effects of normal activities also are blunted, creating a state called anhedonia (a reduced ability to experience pleasure), a hallmark of prolonged addiction.
The prolonged impairment of the dopamine balance and pleasure circuits in the brain leads to changes in the neurochemical pathways and structures in the reward-related areas of the brain. Micro-structural abnormalities are present in the white matter of several specific brain regions of drug-dependent patients, notably in heroin and cocaine users. These changes drive the intense cravings and compulsive behaviors for substances that increase dopamine levels.
Furthermore, these neurochemical and structural changes persist even after use of the addictive substances stop or the addictive behaviors cease. Specific white matter abnormalities may serve as markers of abstinence duration and reflect recovery from addiction. Current research indicates that it may take as long as three years or more for many of these structural changes to resolve.
Evaluating Addiction Risk and Management
Vulnerability to addiction differs from person to person and is influenced by both environmental factors (home, family, nutrition, peer pressure, availability of drugs, age of drug use and method of administration), experiential factors (stress, chronic pain, PTSD) and genetic risk factors. Researchers estimate that genetic factors account for 40% to 60% of a person’s vulnerability to addiction, (especially alcoholism) including the effects of environment and experience on gene expression and function (epigenetics). Also, individuals with co-existing mental disorders are at greater risk of drug abuse and addiction than the general population.
“Reward Deficiency Syndrome”
Those individuals with inadequate levels of dopamine in the reward center of their brain due to genetic or environmental circumstances, experience an innate drive to respond to chemicals and activities that increase their dopamine levels. As a consequence, individuals with inadequate dopamine levels experience an exaggerated positive response to drugs and activities that increase their low dopamine levels. This is the basis of the “Reward Deficiency Syndrome (RDS),” the condition of sub-optimal dopamine levels in the reward center (the NAc) and related brain areas.
It is further recognized that a variety of activities increase dopamine levels, including behaviors such as sex, binge eating, gambling, thrill-seeking, computer gaming and even expressing strong anger. The dopamine drive inherent in these behaviors is believed to contribute to their addictive potential, especially in those people who may have genetic or environmental circumstances that predispose them to low dopamine levels and/or low amounts of DA receptors.
Understanding how genetic variants, stress and other factors contribute to dopamine levels provides opportunities for alternative approaches to treating addiction and other manifestations of RDS.
See: Reward Deficiency Syndrome (RDS)
Treatment of Addiction Based on Management of RDS
The challenge in treating addiction is to facilitate the return to neurochemical and structural normalcy as a means of preventing relapse and maintaining a sense of well-being. Medication Assisted Therapy (MAT), the current mainstay of addiction management for some drug addictions including opioids, engages the use of medications such as Suboxone and methadone directed at maintaining dopamine levels. Their effect is to suppress cravings to allow for behavioral methods to reduce environmental risks that drive relapse and provide time for the structural elements in the brain to recover.
Genetic-based Approaches to Management of RDS
Addressing the neurochemical basis of addiction can be a key method treatment. There is good evidence that supports “dopamine homeostasis” as a goal of treatment for RDS. Understanding how genetic variants impact the reward system and dopamine levels provides opportunities for alternative approaches to treating addiction and other RDS-associated conditions. Recent research into the genetics of RDS and addiction now allows for individualized genetic testing for DNA variants that contribute to addiction susceptibility with a simple swab of saliva (the GARS). Targets for treatment include addressing an individual’s specific genetic variants that contribute to addiction susceptibility as a consequence of suboptimal dopamine production, excessive dopamine breakdown or reduced numbers of dopamine receptors.
Restoring dopamine balance has been shown to be effective at reducing RDS symptoms and severity, especially in certain addictions and chronic pain conditions. Dopamine balance can be accomplished through the use of genetically guided neuro-nutrients now available. For example, the use of low doses of a dopamine agonist nutritional supplement such as SynaptaGenX or other supplement variants provides a constant, low level stimulation of the DA receptor system resulting in significant proliferation of D2 receptors, in spite of genetic variants.
Multi-modal Approach
In the management of addiction it is also important to understand the value of a multi-modal approach. For example, naltrexone is a potent blocker of the mu-opioid receptor and effectively reduces opioid and alcohol consumption in those with opioid (OUD) and alcohol use disorders (AUD). While naltrexone is associated with higher compliance with OUD than for AUD and shows benefit especially in the short term, there is evidence that the retention and compliance with naltrexone is not sufficient to characterize adherence as high. Furthermore, naltrexone is associated with higher rates of concurrent non-opioid substance use like cocaine. One reason that explains this is that chronically blocking μ-receptors also chronically down-regulates mesolimbic dopamine release, contributing to low dopamine levels and RDS. Studies show that a combination of naltrexone and a pro-dopamine regulator neuro-nutrient (such as SynaptaGenX) significantly prevents opioid relapse.
Similarly regarding buprenorphine (i.e. Suboxone) and dopaminergic function, acute doses of buprenorphine increase dopamine release, whereas chronic dosing leads to reduced dopamine release and dysregulation. Again, the goal of treatment for any Substance Use Disorder (SUD) is to restore dopamine homeostasis. It is recommended that a pro-dopamine regulator neuro-nutrient (such as SynaptaGenX) also be added to buprenorphine management of OUD.
See:
Reward Deficiency Syndrome: Genetic Testing
Genetic Testing: Individual DNA Alleles
Environment-based Approaches to Management of RDS
Environmental factors, especially chronic pain and stress, have a tremendous impact on the reward system and dopamine levels. Adequately treating pain, reducing stress and the manifestations of stress are important in the treatment of RDS. Mindful exercises including meditation, Tai Chi, yoga and deep relaxation techniques are useful modalities in managing stress and restore dopamine levels. Not to be overlooked, aerobic exercise reduces stress and positively impacts most RDS-associated conditions and is strongly recommended.
See: Using the Mind & Dopamine Enhancement
Dopamine-boosting dietary approaches designed to enhance dopamine have been proposed. The use of nutrigenomic supplements based on genetic testing are also advised, (see above).
See: Diet & Dopamine
Experiential Factors in Management of RDS
Memory plays a cornerstone role in many RDS-associated conditions and can be a major contributing influence in behaviors related to RDS. Hypnosis is a very useful tool that can be incorporated to effectively “re-write” memories and defuse their role in maladaptive behaviors and emotions.
See:
Using the Mind
Hypnosis
Dopamine Enhancement
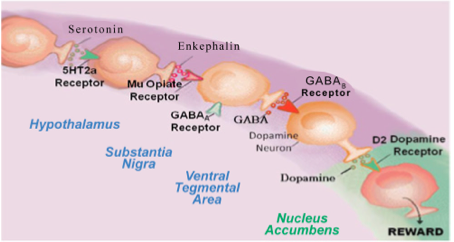
The Brain Reward Cascade
The brain reward cascade starts in the hypothalamus of the brain sitting in the midbrain called the mesolimbic system where serotonin acts as the neurotransmitter activating the enkephalins (one type of brain endorphin); the enkephalins are released in the hypothalamus and stimulate mu receptors in another part of the brain called substania nigra that contains the neurotransmitter GABA (an inhibitory neurotransmitter) that stimulates GABA-B receptors that projects to the ventral tegmental area (VTA) brain region where dopamine neurons are inhibited to allow for just the right amount of dopamine to be released at the nucleus accumbens (reward site of brain).
Reward Deficiency Syndrome (RDS)
Associated with the new definition and understanding of addiction as related to reward deficiency is a major paradigm shift in our approaches to treatment. The following articles are available to provide further understanding of the nature of Reward Deficiency Syndrome and how to treat it.
RDS – Overview
- The Addictive Brain – All Roads Lead to Dopamine – 2012
- Hatching the behavioral addiction egg – Reward Deficiency Solution System – 2014
- Sex, Drugs, and Rock ‘N’ Roll – Hypothesizing Common Mesolimbic Activation as a Function of Reward Gene Polymorphisms – 2012
- “Dopamine homeostasis” requires balanced polypharmacy – Issue with destructive, powerful dopamine agents to combat America’s drug epidemic
- Neurodynamics of relapse prevention-neuronutrient approach to outpatient DUI offenders
- Genetic Addiction Risk Testing Coupled with Pro Dopamine Homeostasis
- Pro-dopamine regulator, KB220Z, attenuates hoarding and shopping behavior in a female, diagnosed with SUD and ADHD
- Neuro-Nutrient Effects on Weight Loss in Carbohydrate Bingers – an open clinical trial
- Enkephalinase Inhibition – Regulation of Ethanol Intake in Genetically Predisposed Mice
- The D2 dopamine receptor gene as a determinant of reward deficiency syndrome – 1996
- Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. – PubMed – NCBI
- Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS) – a commentary – 2008
- Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. – PubMed – NCBI
- Association of polymorphisms of dopamine D2 receptor (DRD2), and dopamine transporter (DAT1) genes with schizoid:avoidant behaviors (SAB). – PubMed – NCBI
- Role of dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse – 2011
- Reward deficiency syndrome: genetic aspects of behavioral disorders. – PubMed – NCBI
- The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes’ theorem. – PubMed – NCBI
- Delayed P300 latency correlates with abnormal Test of Variables of Attention (TOVA) in adults and predicts early cognitive decline in a clinical se… – PubMed – NCBI
RDS – Addiction
- The Addictive Brain – All Roads Lead to Dopamine – 2012
- Hatching the behavioral addiction egg – Reward Deficiency Solution System – 2014
- “Dopamine homeostasis” requires balanced polypharmacy – Issue with destructive, powerful dopamine agents to combat America’s drug epidemic
- Neurodynamics of relapse prevention-neuronutrient approach to outpatient DUI offenders
- The D2 dopamine receptor gene as a determinant of reward deficiency syndrome – 1996
- Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. – PubMed – NCBI
- Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS) – a commentary – 2008
- Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. – PubMed – NCBI
- Analysis of Evidence for the Combination of Pro-dopamine Regulator (KB220PAM) and Naltrexone to Prevent Opioid Use Disorder Relapse – 2018
- Inhibitory Plasticity of Mesocorticolimbic Circuits in Addiction and Mental Illness. – PubMed – NCBI
RDS – ADD
- Attention-deficit-hyperactivity disorder and reward deficiency syndrome – 2008
- Reward Deficiency Syndrome – Attentional Arousal Subtypes, Limitations of Current Diagnostic Nosology, and Future Research – 2015
- Neurogenetic interactions and aberrant behavioral co-morbidity of attention deficit hyperactivity disorder (ADHD) -dispelling myths – 2005
- Epigenetics in Developmental Disorder – ADHD and Endophenotypes
- Low Dopamine Function in Attention Deficit:Hyperactivity Disorder – Should Genotyping Signify Early Diagnosis in Children? – 2014
- Enhancement of attention processing by Kantroll in healthy humans: a pilot study. – PubMed – NCBI
- Pro-dopamine regulator, KB220Z, attenuates hoarding and shopping behavior in a female, diagnosed with SUD and ADHD
- Delayed P300 latency correlates with abnormal Test of Variables of Attention (TOVA) in adults and predicts early cognitive decline in a clinical se… – PubMed – NCBI
RDS – Exercise
- Physical Exercise Interventions for Drug Addictive Disorders – 2017
- Basal ganglia dysfunction contributes to physical inactivity in obesity – 2017
- Running from Disease – Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes – 2017
RDS – Gaming
RDS – Genetics
- Multilocus Genetic Composite Reflecting Dopamine Signaling Capacity Predicts Reward Circuitry Responsivity 2012
- Genetic Addiction Risk Score (GARS) – Testing For Polygenetic Predisposition and Risk to Reward Deficiency Syndrome (RDS) – 2011
- Neurogenetic Impairments of Brain Reward Circuitry Links to Reward Deficiency Syndrome (RDS) – Potential Nutrigenomic Induced Dopaminergic Activation
- Genetic Addiction Risk Testing Coupled with Pro Dopamine Homeostasis
- The D2 dopamine receptor gene as a determinant of reward deficiency syndrome – 1996
- Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. – PubMed – NCBI
- Association of polymorphisms of dopamine D2 receptor (DRD2), and dopamine transporter (DAT1) genes with schizoid:avoidant behaviors (SAB). – PubMed – NCBI
- Reward deficiency syndrome: genetic aspects of behavioral disorders. – PubMed – NCBI
- The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes’ theorem. – PubMed – NCBI
- Genetic addiction risk score (GARS), a predictor of vulnerability to opioid dependence – 2018
- Common Neurogenetic Diagnosis and Meso-Limbic Manipulation of Hypodopaminergic Function in Reward Deficiency Syndrome (RDS) – Changing the Recovery Landscape – 2017
- Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation – 2013
RDS – Food Addiction & Obesity
- Reward Deficiency Syndrome Studies of KB220 Variants
- Mood, food, and obesity
- Dopamine and glucose, obesity, and reward deficiency syndrome – 2014
- Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. – PubMed – NCBI
- “Liking” and “Wanting” Linked to Reward Deficiency Syndrome (RDS) – Hypothesizing Differential Responsivity in Brain Reward Circuitry – 2012
- Neuro-Genetics of Reward Deficiency Syndrome (RDS) as the Root Cause of “Addiction Transfer”- 2011
- Physical Exercise Interventions for Drug Addictive Disorders – 2017
- Basal ganglia dysfunction contributes to physical inactivity in obesity – 2017
- Running from Disease – Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes – 2017
- Incorporating food addiction into disordered eating – the disordered eating food addiction nutrition guide (DEFANG) – 2017
- Do Dopaminergic Impairments Underlie Physical Inactivity in People with Obesity? – 2016
- Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic – 2016
- A meta-analysis of the relationship between brain dopamine receptors and obesity – a matter of changes in behavior rather than food addiction? – 2016
- Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation – 2013
- Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) … – PubMed – NCBI
- LOW DOPAMINE D2 RECEPTOR INCREASES VULNERABILITY TO OBESITY VIA REDUCED PHYSICAL ACTIVITY NOT INCREASED APPETITIVE MOTIVATION – 2016
- Dopamine and glucose, obesity, and reward deficiency syndrome – 2014
- Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. – PubMed – NCBI – 2018
- Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. – PubMed – NCBI
- LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. – PubMed – NCBI
- Basal ganglia dysfunction contributes to physical inactivity in obesity – 2017
- Running from Disease – Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes – 2017
- Incorporating food addiction into disordered eating – the disordered eating food addiction nutrition guide (DEFANG) – 2017
- Do Dopaminergic Impairments Underlie Physical Inactivity in People with Obesity? – 2016
- Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) … – PubMed – NCBI
- LOW DOPAMINE D2 RECEPTOR INCREASES VULNERABILITY TO OBESITY VIA REDUCED PHYSICAL ACTIVITY NOT INCREASED APPETITIVE MOTIVATION – 2016
- Dopamine and glucose, obesity, and reward deficiency syndrome – 2014
- Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. – PubMed – NCBI – 2018
- Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. – PubMed – NCBI
- LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. – PubMed – NCBI
RDS – Chronic Pain
- Hypothesizing that brain reward circuitry genes are genetic antecedents of pain sensitivity and critical diagnostic and pharmacogenomic – 2009
- A Multi-Locus Approach to Treating Fibromyalgia by Boosting Dopaminergic Activity in the Meso-Limbic System of the Brain – 2014
- Love as a Modulator of Pain – 2017
- Comorbidity of alcohol use disorder and chronic pain – Genetic influences on brain reward and stress systems – 2017
- Reward Circuitry Plasticity in Pain Perception and Modulation – 2017
- Modulation of pain, nociception, and analgesia by the brain reward center – 2016
- Pharmacology of enkephalinase inhibitors: animal and human studies. – PubMed – NCBI 198
- Analgesic properties of enkephalinase inhibitors: animal and human studies. – PubMed – NCBI 1985
- DL-phenylalanine markedly potentiates opiate analgesia – an example of nutrient:pharmaceutical up-regulation of the endogenous analgesia system. – PubMed – NCBI – 2000
- Iatrogenic opioid dependence is endemic and legal – Genetic addiction risk score (GARS) with electrotherapy a paradigm shift in pain treatment programs – 2013
- Mesolimbic dopamine signaling in acute and chronic pain – implications for motivation, analgesia, and addiction – 2016
- Microglia Disrupt Mesolimbic Reward Circuitry in Chronic Pain Positive emotions and brain reward circuits in chronic pain – 2016
- The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain – 2016
- Dopamine and Pain Sensitivity – Neither Sulpiride nor Acute Phenylalanine and Tyrosine Depletion Have Effects on Thermal Pain Sensations in Healthy Volunteers – 2013
- Dopamine Precursor Depletion Influences Pain Affect Rather than Pain Sensation – 2014
- Insurance Companies Fighting the Peer Review Empire without any Validity – 2018
- The dynamic interaction between pain and opioid misuse-2018
RDS – PTSD
- Neuro-psychopharmacogenetics and Neurological Antecedents of Posttraumatic Stress Disorder – Unlocking the Mysteries of Resilience and Vulnerability – 2010
- Diagnosis and Healing In Veterans Suspected of Suffering from Post-Traumatic Stress Disorder (PTSD) Using Reward Gene Testing and RewardCircuitry Natural Dopaminergic Activation-2012
- Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients – Role of enhanced brain reward functional connectivity and homeostasis redeeming joy – 2015
RDS – Sleep
RDS – Treatment
RDS Treatment – Overview
- Clinically Combating Reward Deficiency Syndrome (RDS) with Dopamine Agonist Therapy as a Paradigm Shift – Dopamine for Dinner? 2015
- Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS)
- Pro-dopamine regulator, KB220Z, attenuates hoarding and shopping behavior in a female, diagnosed with SUD and ADHD
- Neuro-Nutrient Effects on Weight Loss in Carbohydrate Bingers – an open clinical trial
- Enkephalinase Inhibition – Regulation of Ethanol Intake in Genetically Predisposed Mice
RDS Treatment – Meditation
RDS Treatment – Music Therapy
RDS Treatment – SynaptaGenX
SynaptaGenX – Overviews
- Synaptamine – brief summary
- Hypothesizing That Neuropharmacological and Neuroimaging Studies of Glutaminergic-Dopaminergic Optimization Complex (KB220Z) – 2017
- GLOBAL OPIOID EPIDEMIC – DOOMED TO FAIL WITHOUT GENETICALLY BASED PRECISION ADDICTION MEDICINE (PAM™) – LESSONS LEARNED FROM AMERICA – 2018
SynaptaGenX – Buptrenorphine
SynaptaGenX – Fibromyalgia
SynaptaGenX – Lucid Nightmares
- Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients – Role of enhanced brain reward functional connectivity and homeostasis redeeming joy – 2015
- Using the Neuroadaptagen KB200zTM to Ameliorate Terrifying, Lucid Nightmares in RDS Patients – the Role of Enhanced, Brain- Reward, Functional Connectivity and Dopaminergic Homeostasis – 2015
SynaptaGenX – PTSD
- Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients – Role of enhanced brain reward functional connectivity and homeostasis redeeming joy – 2015
- Diagnosis and Healing In Veterans Suspected of Suffering from Post-Traumatic Stress Disorder (PTSD) Using Reward Gene Testing and RewardCircuitry Natural Dopaminergic Activation-2012
SynaptaGenX – Sleep
.
Emphasis on Education
Accurate Clinic promotes patient education as the foundation of it’s medical care. In Dr. Ehlenberger’s integrative approach to patient care, including conventional and complementary and alternative medical (CAM) treatments, he may encourage or provide advice about the use of supplements. However, the specifics of choice of supplement, dosing and duration of treatment should be individualized through discussion with Dr. Ehlenberger. The following information and reference articles are presented to provide the reader with some of the latest research to facilitate evidence-based, informed decisions regarding the use of conventional as well as CAM treatments.
For medical-legal reasons, access to these links is limited to patients enrolled in an Accurate Clinic medical program.
Should you wish more information regarding any of the subjects listed – or not listed – here, please contact Dr. Ehlenberger. He has literally thousands of published articles to share on hundreds of topics associated with pain management, weight loss, nutrition, addiction recovery and emergency medicine. It would take years for you to read them, as it did him.
For more information, please contact Accurate Clinic.
Supplements recommended by Dr. Ehlenberger may be purchased commercially online or at Accurate Clinic.
Please read about our statement regarding the sale of products recommended by Dr. Ehlenberger.
Accurate Supplement Prices
.