The influence of the gut microbiome on many physiological and psychological processes including pain is complex. It involves bi-directional communication between the gut and the brain, the hormone, immune and endocannabinoid systems as well as microbial production of neuroactive compounds.
It has been shown that the microbiome of people suffering from various medical conditions differs significantly from that of healthy controls. Research suggests that altering a person’s microbiome through the use of probiotics, prebiotics, or dietary change can alleviate the symptoms and course of many medical conditions including pain.
See: Gut Microbiota
The Gut Microbiota and Pain
Growing evidence indicates that the gut microbiota plays a significant role in visceral pain (pain originating in the internal organs of the body), as well as in abdominal pain, headache, inflammatory pain and neuropathic pain (NP). Additionally, microbiota-gut-brain communication has been implicated in Alzheimer’s disease, Parkinson’s disease, depression as well as pain. Interestingly, the microbiota has also been linked to the development of opioid tolerance.
Recent research indicates that the development of NP is related to the evolution of peripheral and central sensitization, in which the nervous system becomes “sensitized,” resulting in the magnification of the pain experience as well as the transitioning of acute to chronic pain. Treatment options for peripheral sensitization (PS) and central sensitization (CS) are limited and represent the holy grail of pain management.
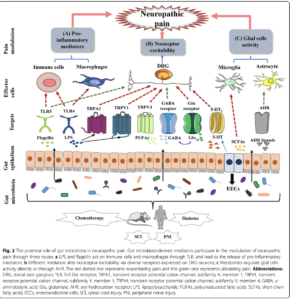
Both (PS) and (CS) are driven by neuroinflammation, in which nerves become sensitized by exposure to pro-inflammatory chemicals (cytokines and chemokines) released by immune cells in the nervous system, including macrophages and glial cells.
Bacteria in the gut are able to affect neuroinflammation by decreasing the production of pro-inflammatory cytokines. As such, alteration of gut microbiota could lead to the up-regulation and down-regulation of cytokines and chemokines which may affect the occurrence of NP.
Gut microbiota-derived mediators participate in the modulation of neuropathic pain through three routes:
- LPS and flagellin act on immune cells and macrophages through Toll-Like Receptors (TLR), and lead to the release of pro-inflammatory mediators;
- Different mediators alter nociceptor excitability via diverse receptors expressed on DRG neurons;
- Metabolites regulate glial cells activity directly or through aryl hydrocarbon receptors (AHR).
Conclusion
The impact of the gut microbiome on health and disease is currently one of the most stimulating areas of medicine. Learning more about the gut microbiome may provide new treatment options for many conditions and diseases currently resistant to effective management including fibromyalgia, irritable bowel disease, Crohn’s, ulcerative colitis and autism.
The medical world is just beginning to learn about the gut microbiome, its role in dietary intake and disease development, and the effect of probiotic supplementation on various disease states.
References:
Gut Microbiota
Gut Microbiota – Overviews
- The Gut Microbiome- What we do and don’t know – 2015
- The role of the microbiome for human health- from basic science to clinical applications – 2018
- Influence of diet on the gut microbiome and implications for human health – 2017
- Man and the Microbiome- A New Theory of Everything? – PubMed 2019
Gut Microbiota – Pain
- Stress and the Microbiota–Gut–Brain Axis in Visceral Pain – Relevance to Irritable Bowel Syndrome – 2016
- The Role of the Gastrointestinal Microbiota in Visceral Pain – PubMed 2017
- Gut microbiota regulates neuropathic pain – potential mechanisms and therapeutic strategy – 2020
Gut Microbiota – Fibromyalgia
Gut Microbiota – Psychiatric Disorders
Gut Microbiota – Small Intestinal Bacterial Overgrowth (SIBO)
Probiotics
Probiotics – Overviews
Probiotics – Foods
Probiotics – IBD (Inflammatory Bowel Diseases)
Probiotics – IBS
- Stress and the Microbiota–Gut–Brain Axis in Visceral Pain – Relevance to Irritable Bowel Syndrome – 2016
- Adherence to the pro-inflammatory diet in relation to prevalence of irritable bowel syndrome – 2019
- The Evolving Role of Gut Microbiota in the Management of Irritable Bowel Syndrome – An Overview of the Current Knowledge – 2020
Probiotics – Infections
- Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055 – 2019
- Probiotics and Paraprobiotics in Viral Infection – Clinical Application and Effects on the Innate and Acquired Immune Systems – 2018
- Probiotics in respiratory virus infections – 2014
Probiotics – Pain
- Lactobacillus paracasei S16 Alleviates Lumbar Disc Herniation by Modulating Inflammation Response and Gut Microbiota – 2021.pdf
- Visceral pain – gut microbiota, a new hope? – 2019